Abstract
The administration of antineoplastic agents directly into the peritoneal cavity as treatment of localised cancer is based on sound pharmacokinetic principles. This unique technique has to the potential to optimise outcome in settings where preclinical and clinical data suggest that cytotoxicity of a specific drug against a particular tumour type is enhanced by either increasing the drug concentration or duration of exposure.
Phase I trials have confirmed the safety and pharmacokinetic advantage for a number of agents delivered by the intraperitoneal relative to the systemic route, including cisplatin (10- to 20-fold advantage for regional delivery), carboplatin (10- to 20-fold advantage), and paclitaxel (1000-fold advantage). In phase II rials, performed mostly in patients with ovarian cancer, this approach has achieved objective responses in settings where intravenous drug delivery has not achieved the desired effect (e.g. surgically documented complete response using intraperitoneal cisplatin as second-line therapy of ovarian cancer). Phase III trials employing intraperitoneal cisplatin as initial treatment of small volume advanced ovarian cancer have demonstrated that regional therapy results in a modest, but statistically significant, improvement in both progression-free and overall survival compared to intravenous cisplatin.
Further exploration of this novel method of treatment, including the conduct of definitive randomised phase III clinical trials, is indicated in ovarian cancer and in other tumour types where clinical manifestations are principally localised to the peritoneal cavity.



Similar content being viewed by others
References
Weisberger AS, Levine B, Storaasli JP. Use of nitrogen mustard in treatment of serous effusions of neoplastic origin. J Am Med Assoc 1955; 159: 1704–7
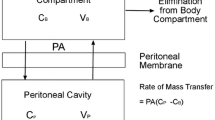
Dedrick RL, Myers CE, Bungay PM, et al. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep 1978; 62: 1–9
Alberts DS, Young L, Mason N, et al. In vitro evaluation of anticancer drugs against ovarian cancer at concentrations achievable by intraperitoneal administration. Semin Oncol 1985; 12 (3 Suppl. 4): 38–42
Markman M. Intraperitoneal therapy for treatment of malignant disease principally confined to the peritoneal cavity. Crit Rev Oncol Hematol 1993; 14: 15–28
Markman M. Intraperitoneal chemotherapy. Crit Rev Oncol Hematol 1999; 31: 239–46
Howell SB, Pfeifle CE, Wung WE, et al. Intraperitoneal cisplatin with systemic thiosulfate protection. Ann Intern Med 1982; 97: 845–51
Casper ES, Kelsen DP, Alcock NW, et al. Ip cisplatin in patients with malignant ascites: pharmacokinetics evaluation and comparison with the iv route. Cancer Treat Rep 1983; 67: 325–38
DeGregorio MW, Lum BL, Holleran WM, et al. Preliminary observations of intraperitoneal carboplatin pharmacokinetics during a phase I study of the Northern California Oncology Group. Cancer Chemother Pharmacol 1986; 18: 235–38
Elferink F, van der Vijgh WJF, ten Bokkel Huinink WW, et al. Pharmacokinetics of carboplatin after intraperitoneal administration. Cancer Chemother Pharmacol 1988; 21: 57–60
Markman M, Rowinsky E, Hakes T, et al. Phase 1 trial of intraperitoneal taxol: a Gynecologic Oncology Group Study. J Clin Oncol 1992; 10: 1485–91
Francis P, Rowinsky E, Schneider J, et al. Phase 1 feasibility and pharmacologic study of weekly intraperitoneal paclitaxel: a Gynecologic Oncology Group pilot study. J Clin Oncol 1995; 13: 2961–7
Ozols RF, Young RC, Speyer JL, et al. Phase 1 and pharmacological studies of adriamycin administered intraperitoneally to patients with ovarian cancer. Cancer Res 1982; 42: 4265–9
Markman M, George M, Hakes T, et al. Phase 2 trial of intraperitoneal mitoxantrone in the management of refractory ovarian carcinoma. J Clin Oncol 1990; 8: 146–50
Monk BJ, Surwit EA, Alberts DS, et al. Intraperitoneal mitomycin C in the treatment of peritoneal carcinomatosis following second-look surgery. Semin Oncol 1988; 15: 27–31
Cannistra SA. Cancer of the ovary. N Engl J Med 1993; 329: 1550–9
Markman M, Reichman B, Hakes T, et al. Responses to secondline cisplatin-based intraperitoneal therapy in ovarian cancer: influence of a prior response to intravenous cisplatin. J Clin Oncol 1991; 9: 1801–5
Markman M, Berek JS, Blessing JA, et al. Characteristics of patients with small-volume residual ovarian cancer unresponsive to cisplatin-based IP chemotherapy: lessons learned from a Gynecologic Oncology Group phase II trial of IP cisplatin and recombinant alpha-interferon. Gynecol Oncol 1992; 45: 3–8
Markman M, Blessing JA, Major F, et al. Salvage intraperitoneal therapy of ovarian cancer employing cisplatin and etoposide: a Gynecologic Oncology Group Study. Gynecol Oncol 1993; 50: 191–5
West GW, Weichselbau R, Little JB. Limited penetration of methotrexate into human osteosarcoma spheroids as a proposed model for solid tumor resistance to adjuvant chemotherapy. Cancer Res 1980; 40: 3665–8
Durand RE. Flow cytometry studies of intracellular adriamycin in multicell spheroids in vitro. Cancer Res 1981; 41: 3495–8
Ozols RF, Locker GY, Doroshow JH, et al. Pharmacokinetics of adriamycin and tissue penetration in murine ovarian cancer. Cancer Res 1979; 39: 3209–14
Nederman T, Carlsson J. Penetration and binding of vinblastine and 5-fluorouracil in cellular spheroids. Cancer Chemother Pharmacol 1984; 13: 131–5
Los G, Mutsaers PHA, van der Vijgh WJF, et al. Direct diffusion of cis-diamminedichloroplatinum(II) in intraperitoneal rat tumors after intraperitoneal chemotherapy: a comparison with systemic chemotherapy. Cancer Res 1989; 49: 3380–4
Markman M, Brady MF, Spirtos NM, et al. Phase II trial of intraperitoneal paclitaxel in carcinoma of the ovary, tube, and peritoneum: a Gynecologic Oncology Group Study. J Clin Oncol 1998; 16: 2620–4
Berek JS, Markman M, Stonebraker B, et al. Intraperitoneal interferon-a in residual ovarian carcinoma: a phase II Gynecologic Oncology Group Study. Gynecol Oncol 1999; 75: 10–14
Pujade-Lauraine E, Guastalla J-P, Colombo N, et al. Intraperitoneal recombinant interferon gamma in ovarian cancer patients with residual disease at second-look laparotomy. J Clin Oncol 1996; 14: 343–50
Edwards RP, Gooding W, Lembersky BC, et al. Comparison of toxicity and survival following intraperitoneal recombinant interleukin-2 for persistent ovarian cancer after platinum: twenty-four-hour versus 7-day infusion. J Clin Oncol 1997; 15: 3399–3407
Markman M. Is there a role for intraperitoneal therapy in the management of gastrointestinal malignancies? Cancer Invest 1995; 13(6): 625–8
Markman M, Cleary S, Pfeifle CE, et al. Cisplatin administered by the intracavitary route as treatment for malignant mesothelioma. Cancer 1986; 58: 18–21
Markman M, Kelsen D. Efficacy of cisplatin-based intraperitoneal chemotherapy as treatment of malignant peritoneal mesothelioma. J Cancer Res Clin Oncol 1992; 118: 547–50
Sugarbaker PH, Zhu B-W, Sese GB, et al. Peritoneal carcinomatosis from appendiceal cancer: results in 69 patients treated by cytoreductive surgery and intraperitoneal chemotherapy. Dis Colon Rectum 1993; 36: 323–9
Howell SB, Zimm S, Markman M, et al. Long term survival of advanced refractory ovarian carcinoma patients with small-volume disease treated with intraperitoneal chemotherapy. J Clin Oncol 1987; 5: 1607–12
Markman M, Reichman B, Hakes T, et al. Impact on survival of surgically-defined favorable responses to salvage intraperitoneal chemotherapy in small volume residual ovarian cancer. J Clin Oncol 1992; 10: 1479–84
Piver MS, Recio FO, Baker TR, et al. Evaluation of survival after second-line intraperitoneal cisplatin-based chemotherapy for advanced ovarian cancer. Cancer 1994; 73: 1693–8
Barakat RR, Almadrones L, Venkatraman ES, et al. A phase II trial of intraperitoneal cisplatin and etoposide as consolidation therapy in patients with stage II-IV epithelial ovarian cancer following negative surgical assessment. Gynecol Oncol 1998; 69: 17–22
Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 1996; 335: 1950–5
Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 1998; 351: 1451–67
McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 1996; 334: 1–6
Markman M, Bundy BN, Alberts DS, et al. Randomized phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume small III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol 2001; 19: 1001–7
Shapiro F, Schneider J, Markman M, et al. High-intensity intravenous cyclophosphamide and cisplatin, interim surgical debulking, and intraperitoneal cisplatin in advanced ovarian carcinoma: a pilot trial with ten-year follow-up. Gynecol Oncol 1997; 67: 39–45
Sugarbaker PH, Gianola FJ, Speyer JC, et al. Prospective, randomized trial of intravenous versus intraperitoneal 5-fluorouracil in patients with advanced primary colon or rectal cancer. Surgery 1985; 98: 414–21
Hagiwara A, Takahashi T, Kojima O, et al. Prophylaxis with carbon-adsorbed mitomycin against peritoneal recurrence of gastric cancer. Lancet 1992; 339: 629–31
Rosen HR, Jatzko G, Repse S, et al. Adjuvant intraperitoneal chemotherapy with carbon-adsorbed mitomycin in patients with gastric cancer: results of a randomized multicenter trial of the Austrian Working Group for Surgical Oncology. J Clin Oncol 1998; 16: 2733–38
Wansik Y, Whang I, Suh I, et al. Prospective randomized trial of early postoperative intraperitoneal chemotherapy as an adjuvant to resectable gastric cancer. Ann Surg 1998; 228: 347–54
Los G, Verdegaal EME, Mutsaers PHA, et al. Pentetration of carboplatin and cisplatin into rat peritoneal tumor nodules after intraperitoneal chemotherapy. Cancer Chemother Pharmacol 1991; 28: 159–65
Markman M, Reichman B, Hakes T, et al. Evidence supporting the superiority of intraperitoneal cisplatin compared to intraperitoneal carboplatin for salvage therapy of small volume residual ovarian cancer. Gynecol Oncol 1993; 50: 100–4
Dedrick RL, Flessner MF. Pharmacokinetic problems in peritoneal drug administration: tissue penetration and surface exposure. J Natl Cancer Inst 1997; 89: 480–7
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Markman, M. Intraperitoneal Drug Delivery of Antineoplastics. Drugs 61, 1057–1065 (2001). https://doi.org/10.2165/00003495-200161080-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200161080-00003