Abstract
Diabetes mellitus is a major health risk in many countries, and the incidence rates are increasing. Diverse therapeutic agents are applied to treat this condition. Since 1960, numerous mathematical models have been developed to describe the glucose-insulin system, analyse data from diagnostic tests and quantify drug effects. This review summarizes the present state-of-the-art in diabetes modelling, with a focus on models describing drug effects, and identifies major strengths and limitations of the published models.
For diagnostic purposes, the minimal model has remained the most popular choice for several decades, and numerous extensions have been developed. Use of the minimal model is limited for applications other than diagnostic tests. More mechanistic models that include glucose-insulin feedback in both directions have been applied. The use of biophase distribution models for the description of drug effects is not always appropriate. More recently, the effects of various antidiabetic agents on glucose and insulin have been modelled with indirect response models. Such models provide good curve fits and mechanistic descriptions of the effects of antidiabetic drugs on glucose-insulin homeostasis. These and other types of models were used to describe secondary drug effects on glucose and insulin, and effects on ancillary biomarkers. Modelling of disease progression in diabetes can utilize indirect response models as a disturbance of homeostasis.
Future needs are to include glucose-insulin feedback more often, develop mechanistic models for new drug groups, consider dual drug effects on complementary subsystems, and incorporate elements of disease progression.
















Similar content being viewed by others
References
WHO. Fact sheet no. 312: diabetes [online]. Geneva: WHO, 2006. Available from URL: http://www.who.int/mediacentre/factsheets/fs312/en/print.html [Accessed 2008 Apr 10]
LeRoith D. Beta-cell dysfunction and insulin resistance in type 2 diabetes: role of metabolic and genetic abnormalities. Am J Med 2002 Oct 28; 113 Suppl. 6A: 3S–11S
US Centers for Disease Control and Prevention (CDC). National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2005. Atlanta (GA): CDC, 2005
American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care 2008 Mar; 31(3): 596–615
Graham G, Gupta S, Aarons L. Determination of an optimal dosage regimen using a Bayesian decision analysis of efficacy and adverse effect data. J Pharmacokinet Pharmacodyn 2002 Feb; 29(1): 67–88
Boutayeb A, Chetouani A. A critical review of mathematical models and data used in diabetology. Biomed Eng Online 2006; 5: 43
Makroglou A, Li J, Kuang Y. Mathematical models and software tools for the glucose-insulin regulatory system and diabetes: an overview. Appl Numer Math 2006; 56: 559–73
Kansal AR. Modeling approaches to type 2 diabetes. Diabetes Technol Ther 2004 Feb; 6(1): 39–47
Mari A. Mathematical modeling in glucose metabolism and insulin secretion. Curr Opin Clin Nutr Metab Care 2002 Sep; 5(5): 495–501
Pacini G. Mathematical models of insulin secretion in physiological and clinical investigations. Comput Methods Programs Biomed 1994 Jan; 41(3–4): 269–85
Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs 2005; 65(3): 385–411
Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001 Dec 13; 414(6865): 799–806
Mlinar B, Marc J, Janez A, et al. Molecular mechanisms of insulin resistance and associated diseases. Clin Chim Acta 2007 Jan; 375(1–2): 20–35
Raju B, Cryer PE. Maintenance of the postabsorptive plasma glucose concentration: insulin or insulin plus glucagon? Am J Physiol Endocrinol Metab 2005 Aug; 289(2): E181–6
Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet 2005 Apr 9–15; 365(9467): 1333–46
Katz J, Tayek JA. Gluconeogenesis and the Cori cycle in 12-, 20-, and 40-h-fasted humans. Am J Physiol 1998 Sep; 275 (3 Pt 1): E537–42
Kjems LL, Christiansen E, Volund A, et al. Validation of methods for measurement of insulin secretion in humans in vivo. Diabetes 2000 Apr; 49(4): 580–8
Nolan CJ, Madiraju MS, Delghingaro-Augusto V, et al. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes 2006 Dec; 55 Suppl. 2: S16–23
Newsholme P, Keane D, Welters HJ, et al. Life and death decisions of the pancreatic beta-cell: the role of fatty acids. Clin Sci (Lond) 2007 Jan; 112(1): 27–42
Tremblay F, Lavigne C, Jacques H, et al. Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu Rev Nutr 2007 Aug 21; 27: 293–310
Marchetti P, Lupi R, Del Prato S, et al. The pancreatic beta-cell in human type. Nutr Metab Cardiovasc Dis 2006 Mar; 16 Suppl. 1: S3–6
Singh-Franco D, Robles G, Gazze D. Pramlintide acetate injection for the treatment of type 1 and type 2 diabetes mellitus. Clin Ther 2007 Apr; 29(4): 535–62
Ahren B, Larsson H. Impaired glucose tolerance (IGT) is associated with reduced insulin-induced suppression of glucagon concentrations. Diabetologia 2001 Nov; 44(11): 1998–2003
Ludvik B, Thomaseth K, Nolan JJ, et al. Inverse relation between amylin and glucagon secretion in healthy and diabetic human subjects. Eur J Clin Invest 2003 Apr; 33(4): 316–22
Wookey PJ, Lutz TA, Andrikopoulos S. Amylin in the periphery II: an updated mini-review. Sci World J 2006; 6: 1642–55
Young A. Effects on plasma glucose and lactate. Adv Pharmacol 2005; 52: 193–208
Colburn WA, Gottlieb AB, Koda J, et al. Pharmacokinetics and pharmacodynamics of AC137 (25,28,29 tripro-amylin, human) after intravenous bolus and infusion doses in patients with insulin-dependent diabetes. J Clin Pharmacol 1996 Jan; 36(1): 13–24
Kerckhoffs DA, Arner P, Bolinder J. Lipolysis and lactate production in human skeletal muscle and adipose tissue following glucose ingestion. Clin Sci (Lond) 1998 Jan; 94(1): 71–7
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006 Nov 11; 368(9548): 1696–705
Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007 May; 132(6): 2131–57
Deacon CF, Nauck MA, Meier J, et al. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J Clin Endocrinol Metab 2000 Oct; 85(10): 3575–81
Lam TK, Carpentier A, Lewis GF, et al. Mechanisms of the free fatty acid-induced increase in hepatic glucose production. Am J Physiol Endocrinol Metab 2003 May; 284(5): E863–73
Almon RR, Dubois DC, Jin JY, et al. Temporal profiling of the transcriptional basis for the development of corticosteroid-induced insulin resistance in rat muscle. J Endocrinol 2005 Jan; 184(1): 219–32
Koistinen HA, Zierath JR. Regulation of glucose transport in human skeletal muscle. Ann Med 2002; 34(6): 410–8
DeFronzo RA, Gunnarsson R, Bjorkman O, et al. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest 1985 Jul; 76(1): 149–55
Randle PJ, Garland PB, Hales CN, et al. The glucose fatty-acid cycle: its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963 Apr 13; I: 785–9
Delarue J, Magnan C. Free fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care 2007 Mar; 10(2): 142–8
Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest 2002 Jun; 32 Suppl. 3: 14–23
Quon MJ. Advances in kinetic analysis of insulin-stimulated GLUT-4 translocation in adipose cells. Am J Physiol 1994 Jan; 266 (1 Pt 1): E144–50
Kadowaki T, Yamauchi T, Kubota N, et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 2006 Jul; 116(7): 1784–92
Dyck DJ, Heigenhauser GJ, Bruce CR. The role of adipokines as regulators of skeletal muscle fatty acid metabolism and insulin sensitivity. Acta Physiol (Oxf) 2006 Jan; 186(1): 5–16
Koerner A, Kratzsch J, Kiess W. Adipocytokines: leptin — the classical, resistin —the controversial, adiponectin — the promising, and more to come. Best Pract Res Clin Endocrinol Metab 2005 Dec; 19(4): 525–46
DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med 1999 Aug 17; 131(4): 281–303
Wajchenberg BL. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 2007 Apr; 28(2): 187–218
Guo L, Tabrizchi R. Peroxisome proliferator-activated receptor gamma as a drug target in the pathogenesis of insulin resistance. Pharmacol Ther 2006 Jul; 111(1): 145–73
Gastaldelli A, Miyazaki Y, Mahankali A, et al. The effect of pioglitazone on the liver: role of adiponectin. Diabetes Care 2006 Oct; 29(10): 2275–81
Edelman SV, Darsow T, Frias JP. Pramlintide in the treatment of diabetes. Int J Clin Pract 2006 Dec; 60(12): 1647–53
Berman M. Insulin kinetics, models, and delivery schedules. Diabetes Care 1980 Mar-Apr; 3(2): 266–9
Bergman RN, Cobelli C. Minimal modeling, partition analysis, and the estimation of insulin sensitivity. Fed Proc 1980 Jan; 39(1): 110–5
Bolie VW. Coefficients of normal blood glucose regulation. J Appl Physiol 1961 Sep; 16: 783–8
Insel PA, Liljenquist JE, Tobin JD, et al. Insulin control of glucose metabolism in man: a new kinetic analysis. J Clin Invest 1975 May; 55(5): 1057–66
Sherwin RS, Kramer KJ, Tobin JD, et al. A model of the kinetics of insulin in man. J Clin Invest 1974 May; 53(5): 1481–92
Srinivasan R, Kadish AH, Sridhar R. A mathematical model for the control mechanism of free fatty acid-glucose metabolism in normal humans. Comput Biomed Res 1970 Apr; 3(2): 146–65
Bergman RN, Ider YZ, Bowden CR, et al. Quantitative estimation of insulin sensitivity. Am J Physiol 1979 Jun; 236(6): E667–77
Turner RC, Holman RR, Matthews D, et al. Insulin deficiency and insulin resistance interaction in diabetes: estimation of their relative contribution by feedback analysis from basal plasma insulin and glucose concentrations. Metabolism 1979 Nov; 28(11): 1086–96
Himsworth H, Ker R. Insulin-sensitive and insulin insensitive types of diabetes mellitus. Clin Sci 1939; 4: 119–22
Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci 1959 Sep 25; 82: 420–30
Ackerman E, Gatewood LC, Rosevear JW, et al. Model studies of blood-glucose regulation. Bull Math Biophys 1965; 27 Suppl.: 21–37
DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979 Sep; 237(3): E214–23
Andres R, Swerdloff R, Pozesky T, et al. Manual feedback technique for control of glucose concentration. In: Skeggs JL, editor. Automation in analytical chemistry. New York: Medaid, 1966: 486–501
Kjems LL, Volund A, Madsbad S. Quantification of beta-cell function during IVGTT in type II and non-diabetic subjects: assessment of insulin secretion by mathematical methods. Diabetologia 2001 Oct; 44(10): 1339–48
Monzillo LU, Hamdy O. Evaluation of insulin sensitivity in clinical practice and in research settings. Nutr Rev 2003 Dec; 61(12): 397–412
Wallace TM, Matthews DR. The assessment of insulin resistance in man. Diabet Med 2002 Jul; 19(7): 527–34
Tornoe CW, Jacobsen JL, Madsen H. Grey-box pharmacokinetic/pharmacodynamic modelling of a euglycaemic clamp study. J Math Biol 2004 Jun; 48(6): 591–604
Picchini U, Ditlevsen S, De Gaetano A. Modeling the euglycemic hyperinsulinemic clamp by stochastic differential equations. J Math Biol 2006 Nov; 53(5): 771–96
Mager DE, Abernethy DR, Egan JM, et al. Exendin-4 pharmacodynamics: insights from the hyperglycemic clamp technique. J Pharmacol Exp Ther 2004 Nov; 311(2): 830–5
Woodworth JR, Howey DC, Bowsher RR. Establishment of time-action profiles for regular and NPH insulin using spharmacodynamic modeling. Diabetes Care 1994 Jan; 17(1): 64–9
Hoenig M, Thomaseth K, Brandao J, et al. Assessment and mathematical modeling of glucose turnover and insulin sensitivity in lean and obese cats. Domest Anim Endocrinol 2006 Nov; 31(4): 373–89
Bergman RN. Minimal model: perspective from 2005. Horm Res 2005; 64 Suppl. 3: 8–15
Toffolo G, Bergman RN, Finegood DT, et al. Quantitative estimation of beta cell sensitivity to glucose in the intact organism: a minimal model of insulin kinetics in the dog. Diabetes 1980 Dec; 29(12): 979–90
Bergman RN. Pathogenesis and prediction of diabetes mellitus: lessons from integrative physiology. Mt Sinai J Med 2002 Oct; 69(5): 280–90
Cobelli C, Bettini F, Caumo A, et al. Overestimation of minimal model glucose effectiveness in presence of insulin response is due to undermodeling. Am J Physiol 1998 Dec; 275 (6 Pt 1): E1031–6
Ferrannini E, Mari A. How to measure insulin sensitivity. J Hypertens 1998 Jul; 16(7): 895–906
De Gaetano A, Arino O. Mathematical modelling of the intravenous glucose tolerance test. J Math Biol 2000 Feb; 40(2): 136–68
Bergman RN. New concepts in extracellular signaling for insulin action: the single gateway hypothesis. Recent Prog Horm Res 1997; 52: 359–85; discussion 385-7
Bergman RN. Lilly Lecture 1989: toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes 1989 Dec; 38(12): 1512–27
Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 1981 Dec; 68(6): 1456–67
Polonsky KS, Licinio-Paixao J, Given BD, et al. Use of biosynthetic human C-peptide in the measurement of insulin secretion rates in normal volunteers and type I diabetic patients. J Clin Invest 1986 Jan; 77(1): 98–105
Tura A, Ludvik B, Nolan JJ, et al. Insulin and C-peptide secretion and kinetics in humans: direct and model-based measurements during OGTT. Am J Physiol Endocrinol Metab 2001 Nov; 281(5): E966–74
Toffolo G, Campioni M, Basu R, et al. A minimal model of insulin secretion and kinetics to assess hepatic insulin extraction. Am J Physiol Endocrinol Metab 2006 Jan; 290(1): E169–76
Roy A, Parker RS. Dynamic modeling of free fatty acid, glucose, and insulin: an extended “minimal model”. Diabetes Technol Ther 2006 Dec; 8(6): 617–26
Fabietti PG, Canonico V, Federici MO, et al. Control oriented model of insulin and glucose dynamics in type 1 diabetics. Med Biol Eng Comput 2006 Mar; 44(1–2): 69–78
Derouich M, Boutayeb A. The effect of physical exercise on the dynamics of glucose and insulin. J Biomech 2002 Jul; 35(7): 911–7
Cobelli C, Pacini G, Toffolo G, et al. Estimation of insulin sensitivity and glucose clearance from minimal model: new insights from labeled IVGTT. Am J Physiol 1986 May; 250 (5 Pt 1): E591–8
Cobelli C, Caumo A, Omenetto M. Minimal model SG overestimation and SI underestimation: improved accuracy by a Bayesian two-compartment model. Am J Physiol 1999 Sep; 277 (3 Pt 1): E481–8
Hovorka R, Shojaee-Moradie F, Carroll PV, et al. Partitioning glucose distribution/transport, disposal, and endogenous production during IVGTT. Am J Physiol Endocrinol Metab 2002 May; 282(5): E992–1007
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985 Jul; 28(7): 412–9
Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004 Jun; 27(6): 1487–95
Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998 Dec; 21(12): 2191–2
Chang AM, Smith MJ, Bloem CJ, et al. Limitation of the homeostasis model assessment to predict insulin resistance and beta-cell dysfunction in older people. J Clin Endocrinol Metab 2006 Feb; 91(2): 629–34
Li J, Kuang Y, Li B. Analysis of IVGTT glucose-insulin interaction models with time delay. Discrete Contin Dynam Systems Series B 2001; 1(1): 103–24
Silber HE, Jauslin PM, Frey N, et al. An integrated model for glucose and insulin regulation in healthy volunteers and type 2 diabetic patients following intravenous glucose provocations. J Clin Pharmacol 2007 Sep; 47(9): 1159–71
Jauslin PM, Silber HE, Frey N, et al. A disease model describing the regulation of the glucose-insulin system in diabetic patients after IVGTT and OGTT [abstract no. 799; online]. Annual Meeting of the Population Approach Group in Europe; 2005 Jun 16–17; Pamplona. Available from URL: http://www.page-meeting.org/default.asp?keuze=search [Accessed 2008 May 14]
Jauslin PM, Silber HE, Frey N, et al. An integrated glucose-insulin model to describe oral glucose tolerance test data in type 2 diabetics. J Clin Pharmacol 2007 Oct; 47(10): 1244–55
Dansirikul C, Karlsson MO. Insulin secretion and hepatic extraction during euglycemic clamp study: modelling of insulin and C-peptide data [abstract no. 1142; online]. Annual Meeting of the Population Approach Group in Europe; 2007 Jun 13–15; Copenhagen. Available from URL: http://www.page-meeting.org/default.asp?keuze=search [Accessed 2008 May 14]
Gupta N, Hoffman RP, Veng-Pedersen P. Pharmacokinetic/pharmacodynamic differentiation of pancreatic responsiveness in obese and lean children. Biopharm Drug Dispos 2005 Oct; 26(7): 287–94
Gupta N, Hoffman RP, Veng-Pedersen P. Pharmacokinetic/pharmacodynamic insulin-glucose analysis for differentiation of beta-cell function: an 18 month follow-up study in pre-pubertal lean and obese children. Biopharm Drug Dispos 2006 Sep; 27(6): 257–65
Caumo A, Luzi L. First-phase insulin secretion: does it exist in real life? Considerations on shape and function. Am J Physiol Endocrinol Metab 2004 Sep; 287(3): E371–85
Eaton RP, Allen RC, Schade DS, et al. Prehepatic insulin production in man: kinetic analysis using peripheral connecting peptide behavior. J Clin Endocrinol Metab 1980 Sep; 51(3): 520–8
Volund A, Polonsky KS, Bergman RN. Calculated pattern of intraportal insulin appearance without independent assessment of C-peptide kinetics. Diabetes 1987 Oct; 36(10): 1195–202
Watanabe RM, Volund A, Roy S, et al. Prehepatic beta-cell secretion during the intravenous glucose tolerance test in humans: application of a combined model of insulin and C-peptide kinetics. J Clin Endocrinol Metab 1989 Oct; 69(4): 790–7
Christiansen E, Kjems LL, Volund A, et al. Insulin secretion rates estimated by two mathematical methods in pancreas-kidney transplant recipients. Am J Physiol 1998 Apr; 274 (4 Pt 1): E716–25
Lima JJ, Matsushima N, Kissoon N, et al. Modeling the metabolic effects of terbutaline in beta2-adrenergic receptor diplotypes. Clin Pharmacol Ther 2004 Jul; 76(1): 27–37
Dayneka NL, Garg V, Jusko WJ. Comparison of four basic models of indirect pharmacodynamic responses. J Pharmacokinet Biopharm 1993 Aug; 21(4): 457–78
Jusko WJ, Ko HC. Physiologic indirect response models characterize diverse types of pharmacodynamic effects. Clin Pharmacol Ther 1994 Oct; 56(4): 406–19
Sharma A, Jusko WJ. Characterization of four basic models of indirect pharmacodynamic responses. J Pharmacokinet Biopharm 1996 Dec; 24(6): 611–35
Krzyzanski W, Jusko WJ. Mathematical formalism and characteristics of four basic models of indirect pharmacodynamic responses for drug infusions. J Pharmacokinet Biopharm 1998 Aug; 26(4): 385–408
Hanze E, Green B, Duffull SB, et al. The influence of metformin on HbA1c: a PKPD model [poster no. T3379]. AAPS Annual Meeting and Exposition; 2006 Oct 29–Nov 2; San Antonio (TX)
de Winter W, DeJongh J, Post T, et al. A mechanism-based disease progression model for comparison of long-term effects of pioglitazone, metformin and gliclazide on disease processes underlying type 2 diabetes mellitus. J Pharmacokinet Pharmacodyn 2006 Jun; 33(3): 313–43
Koeslag JH, Saunders PT, Terblanche E. A reappraisal of the blood glucose homeostat which comprehensively explains the type 2 diabetes mellitus-syndrome X complex. J Physiol 2003 Jun 1; 549 (Pt 2): 333–46
Ibbini MS, Masadeh MA, Amer MM. A semiclosed-loop optimal control system for blood glucose level in diabetics. J Med Eng Technol 2004 Sep–Oct; 28(5): 189–96
Schlotthauer G, Gamero LG, Torres ME, et al. Modeling, identification and nonlinear model predictive control of type I diabetic patient. Med Eng Phys 2006 Apr; 28(3): 240–50
Parker RS, Doyle 3rd FJ, Peppas NA. A model-based algorithm for blood glucose control in type I diabetic patients. IEEE Trans Biomed Eng 1999 Feb; 46(2): 148–57
Fabietti PG, Canonico V, Orsini-Federici M, et al. Clinical validation of a new control-oriented model of insulin and glucose dynamics in subjects with type 1 diabetes. Diabetes Technol Ther 2007 Aug; 9(4): 327–38
Tiran J, Avruch LI, Albisser AM. A circulation and organs model for insulin dynamics. Am J Physiol 1979 Oct; 237(4): E331–9
Tiran J, Galle KR, Porte Jr D. A simulation model of extracellular glucose distribution in the human body. Ann Biomed Eng 1975 Mar; 3(1): 34–46
Yamasaki Y, Tiran J, Albisser AM. Modeling glucose disposal in diabetic dogs fed mixed meals. Am J Physiol 1984 Jan; 246 (1 Pt 1): E52–61
Porksen N, Hollingdal M, Juhl C, et al. Pulsatile insulin secretion: detection, regulation, and role in diabetes. Diabetes 2002 Feb; 51 Suppl. 1: S245–54
Simon C, Brandenberger G. Ultradian oscillations of insulin secretion in humans. Diabetes 2002 Feb; 51 Suppl. 1: S258–61
Boden G, Ruiz J, Urbain JL, et al. Evidence for a circadian rhythm of insulin secretion. Am J Physiol 1996 Aug; 271 (2 Pt 1): E246–52
Sturis J, Polonsky KS, Mosekilde E, et al. Computer model for mechanisms underlying ultradian oscillations of insulin and glucose. Am J Physiol 1991 May; 260 (5 Pt 1): E801–9
Shapiro ET, Tillil H, Polonsky KS, et al. Oscillations in insulin secretion during constant glucose infusion in normal man: relationship to changes in plasma glucose. J Clin Endocrinol Metab 1988 Aug; 67(2): 307–14
Tolic IM, Mosekilde E, Sturis J. Modeling the insulin-glucose feedback system: the significance of pulsatile insulin secretion. J Theor Biol 2000 Dec 7; 207(3): 361–75
Li J, Kuang Y, Mason CC. Modeling the glucose-insulin regulatory system and ultradian insulin secretory oscillations with two explicit time delays. J Theor Biol 2006 Oct 7; 242(3): 722–35
Berman M, McGuire EA, Roth J, et al. Kinetic modeling of insulin binding to receptors and degradation in vivo in the rabbit. Diabetes 1980 Jan; 29(1): 50–9
Jones RH, Sonksen PH, Boroujerdi MA, et al. Number and affinity of insulin receptors in intact human subjects. Diabetologia 1984 Aug; 27(2): 207–11
de Beaudrap P, Witten G, Biltz G, et al. Mechanistic model of fuel selection in the muscle. J Theor Biol 2006 Sep 7; 242(1): 151–63
Davis EA, Cuesta-Munoz A, Raoul M, et al. Mutants of glucokinase cause hypoglycaemia- and hyperglycaemia syndromes and their analysis illuminates fundamental quantitative concepts of glucose homeostasis. Diabetologia 1999 Oct; 42(10): 1175–86
Sedaghat AR, Sherman A, Quon MJ. A mathematical model of metabolic insulin signaling pathways. Am J Physiol Endocrinol Metab 2002 Nov; 283(5): E1084–101
Sweet IR, Li G, Najafi H, et al. Effect of a glucokinase inhibitor on energy production and insulin release in pancreatic islets. Am J Physiol 1996 Sep; 271 (3 Pt 1): E606–25
Mosekilde E, Jensen KS, Binder C, et al. Modeling absorption kinetics of subcutaneous injected soluble insulin. J Pharmacokinet Biopharm 1989 Feb; 17(1): 67–87
Mosekilde E, Sosnovtseva OV, Holstein-Rathlou NH. Mechanism-based modeling of complex biomedical systems. Basic Clin Pharmacol Toxicol 2005 Mar; 96(3): 212–24
Osterman-Golkar SM, Vesper HW. Assessment of the relationship between glucose and A1c using kinetic modeling. J Diabetes Complications 2006 Sep–Oct; 20(5): 285–94
Beach KW. A theoretical model to predict the behavior of glycosylated hemoglobin levels. J Theor Biol 1979 Dec 7; 81(3): 547–61
Shi K, Tahara Y, Noma Y, et al. The response of glycated albumin to blood glucose change in the circulation in streptozotocin-diabetic rats -comparison of theoretical values with experimental data. Diabetes Res Clin Pract 1992 Sep; 17(3): 153–60
Mager DE, Wyska E, Jusko WJ. Diversity of mechanism-based pharmacodynamic models. Drug Metab Dispos 2003 May; 31(5): 510–8
Danhof M, de Jongh J, De Lange EC, et al. Mechanism-based pharmacokinetic-pharmacodynamic modeling: biophase distribution, receptor theory, and dynamical systems analysis. Annu Rev Pharmacol Toxicol 2007; 47: 357–400
Furchgott RF. The pharmacology of vascular smooth muscle. Pharmacol Rev 1955 Jun; 7(2): 183–265
Sheiner LB, Stanski DR, Vozeh S, et al. Simultaneous modeling of pharmacokinetics and pharmacodynamics: application to d-tubocurarine. Clin Pharmacol Ther 1979 Mar; 25(3): 358–71
Hill AV. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J Physiol (Lond) 1910; 40: IV–VII
Wagner JG. Kinetics of pharmacologic response: I. Proposed relationships between response and drug concentration in the intact animal and man. J Theor Biol 1968 Aug; 20(2): 173–201
Brown SA, Nelson RW, Bottoms GD. Models for the pharmacokinetics and pharmacodynamics of insulin in alloxan-induced diabetic dogs. J Pharm Sci 1987 Apr; 76(4): 295–9
Miyazaki M, Mukai H, Iwanaga K, et al. Pharmacokinetic-pharmacodynamic modelling of human insulin: validity of pharmacological availability as a substitute for extent of bioavailability. J Pharm Pharmacol 2001 Sep; 53(9): 1235–46
Lin S, Chien YW. Pharmacokinetic-pharmacodynamic modelling of insulin: comparison of indirect pharmacodynamic response with effect-compartment link models. J Pharm Pharmacol 2002 Jun; 54(6): 791–800
Rydberg T, Jonsson A, Karlsson MO, et al. Concentration-effect relations of glibenclamide and its active metabolites in man: modelling of pharmacokinetics and pharmacodynamics. Br J Clin Pharmacol 1997 Apr; 43(4): 373–81
Frey N, Laveille C, Paraire M, et al. Population PKPD modelling of the long-term hypoglycaemic effect of gliclazide given as a once-a-day modified release (MR) formulation. Br J Clin Pharmacol 2003 Feb; 55(2): 147–57
Kirchheiner J, Bauer S, Meineke I, et al. Impact of CYP2C9 and CYP2C19 polymorphisms on tolbutamide kinetics and the insulin and glucose response in healthy volunteers. Pharmacogenetics 2002 Mar; 12(2): 101–9
Herman GA, Bergman A, Stevens C, et al. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab 2006 Nov; 91(11): 4612–9
Young MA, Eckland DJ, Eastmond R, et al. Establishing the dose response curve for metabolic control with troglitazone, an insulin action enhancer, in type 2 diabetes patients. Ann Med 1998 Apr; 30(2): 206–12
Bertacca A, Ciccarone A, Cecchetti P, et al. Continually high insulin levels impair Akt phosphorylation and glucose transport in human myoblasts. Metabolism 2005 Dec; 54(12): 1687–93
Gavin 3rd JR, Roth J, Neville Jr DM, et al. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A 1974 Jan; 71(1): 84–8
Bertacca A, Ciccarone A, Cecchetti P, et al. High insulin levels impair intracellular receptor trafficking in human cultured myoblasts. Diabetes Res Clin Pract 2007 Dec; 78(3): 316–23
Melander A, Donnelly R, Rydberg T. Is there a concentration-effect relationship for sulphonylureas? Clin Pharmacokinet 1998 Mar; 34(3): 181–8
Agerso H, Vicini P. Pharmacodynamics of NN2211, a novel long acting GLP-1 derivative. Eur J Pharm Sci 2003 Jun; 19(2–3): 141–50
Osterberg O, Erichsen L, Ingwersen SH, et al. Pharmacokinetic and pharmacodynamic properties of insulin aspart and human insulin. J Pharmacokinet Pharmacodyn 2003 Jun; 30(3): 221–35
Vicini P, Avogaro A, Spilker ME, et al. Epinephrine effects on insulin-glucose dynamics: the labeled IVGTT two-compartment minimal model approach. Am J Physiol Endocrinol Metab 2002 Jul; 283(1): E78–84
Mari A, Sallas WM, He YL, et al. Vildagliptin, a dipeptidyl peptidase-IV inhibitor, improves model-assessed beta-cell function in patients with type 2 diabetes. J Clin Endocrinol Metab 2005 Aug; 90(8): 4888–94
Rostami-Hodjegan A, Peacey SR, George E, et al. Population-based modeling to demonstrate extrapancreatic effects of tolbutamide. Am J Physiol 1998 Apr; 274 (4 Pt 1): E758–71
Kirchheiner J, Brockmoller J, Meineke I, et al. Impact of CYP2C9 amino acid polymorphisms on glyburide kinetics and on the insulin and glucose response in healthy volunteers. Clin Pharmacol Ther 2002 Apr; 71(4): 286–96
Yun HY, Park HC, Kang W, et al. Pharmacokinetic and pharmacodynamic modelling of the effects of glimepiride on insulin secretion and glucose lowering in healthy humans. J Clin Pharm Ther 2006 Oct; 31(5): 469–76
Lee SH, Kwon KI. Pharmacokinetic-pharmacodynamic modeling for the relationship between glucose-lowering effect and plasma concentration of metformin in volunteers. Arch Pharm Res 2004 Jul; 27(7): 806–10
Gopalakrishnan M, Suarez S, Hickey AJ, et al. Population pharmacokinetic-pharmacodynamic modeling of subcutaneous and pulmonary insulin in rats. J Pharmacokinet Pharmacodyn 2005 Aug; 32(3–4): 485–500
Benincosa L, Jusko WJ. Novel method of treatment. Geneva: World Intellectual Property Organization, 1999. Publ. no. W0/2000/027341
Hamrén B, Björk E, Karlsson MO. Mechanism-based pharmacokinetic and pharmacodynamic modelling of tesaglitazar in type 2 diabetes patients [abstract no. 961]. Annual Meeting of the Population Approach Group in Europe; 2006 Jul 14-16; Bruges. Available from URL: http://www.page-meeting.org/default.asp?keuze=search [Accessed 2008 May 14]
Stepensky D, Friedman M, Raz I, et al. Pharmacokinetic-pharmacodynamic analysis of the glucose-lowering effect of metformin in diabetic rats reveals first-pass pharmacodynamic effect. Drug Metab Dispos 2002 Aug; 30(8): 861–8
Hamrén B, Bjork E, Sunzel M, et al. Models for plasma glucose, HbA1c, and hemoglobin interrelationships in patients with type 2 diabetes following tesaglitazar treatment. Clin Pharmacol Ther. Epub 2008 Mar 19
Hong Y, Rohatagi S, Habtemariam B, et al. Population exposure-response modeling of metformin in patients with type 2 diabetes mellitus. J Clin Pharmacol. Epub 2008 Mar 27
Gumbhir-Shah K, Kellerman DJ, DeGraw S, et al. Pharmacokinetics and pharmacodynamics of cumulative single doses of inhaled salbutamol enantiomers in asthmatic subjects. Pulm Pharmacol Ther 1999; 12(6): 353–62
Gumbhir-Shah K, Kellerman DJ, DeGraw S, et al. Pharmacokinetic and pharmacodynamic characteristics and safety of inhaled albuterol enantiomers in healthy volunteers. J Clin Pharmacol 1998 Dec; 38(12): 1096–106
Kveiborg B, Christiansen B, Major-Petersen A, et al. Metabolic effects of beta-adrenoceptor antagonists with special emphasis on carvedilol. Am J Cardiovasc Drugs 2006; 6(4): 209–17
Larsen JL, Bennett RG, Burkman T, et al. Tacrolimus and sirolimus cause insulin resistance in normal Sprague Dawley rats. Transplantation 2006 Aug 27; 82(4): 466–70
Amamoto T, Kumai T, Nakaya S, et al. The elucidation of the mechanism of weight gain and glucose tolerance abnormalities induced by chlorpromazine. J Pharmacol Sci 2006 Oct; 102(2): 213–9
Wirshing DA, Boyd JA, Meng LR, et al. The effects of novel antipsychotics on glucose and lipid levels. J Clin Psychiatry 2002 Oct; 63(10): 856–65
Ramaswamy K, Masand PS, Nasrallah HA. Do certain atypical antipsychotics increase the risk of diabetes? A critical review of 17 pharmacoepidemiologic studies. Ann Clin Psychiatry 2006 Jul–Sep; 18(3): 183–94
Houseknecht KL, Robertson AS, Zavadoski W, et al. Acute effects of atypical antipsychotics on whole-body insulin resistance in rats: implications for adverse metabolic effects. Neuropsychopharmacology 2007 Feb; 32(2): 289–97
Chiu CC, Chen KP, Liu HC, et al. The early effect of olanzapine and risperidone on insulin secretion in atypical-naive schizophrenic patients. J Clin Psychopharmacol 2006 Oct; 26(5): 504–7
Yip C, Lee AJ. Gatifloxacin-induced hyperglycemia: a case report and summary of the current literature. Clin Ther 2006 Nov; 28(11): 1857–66
Yamada C, Nagashima K, Takahashi A, et al. Gatifloxacin acutely stimulates insulin secretion and chronically suppresses insulin biosynthesis. Eur J Pharmacol 2006 Dec 28; 553(1–3): 67–72
Ishiwata Y, Sanada Y, Yasuhara M. Effects of gatifloxacin on serum glucose concentration in normal and diabetic rats. Biol Pharm Bull 2006 Mar; 29(3): 527–31
Zillich AJ, Garg J, Basu S, et al. Thiazide diuretics, potassium, and the development of diabetes: a quantitative review. Hypertension 2006 Aug; 48(2): 219–24
Doyle ME, Egan JM. Pharmacological agents that directly modulate insulin secretion. Pharmacol Rev 2003 Mar; 55(1): 105–31
Cusi K, Kashyap S, Gastaldelli A, et al. Effects on insulin secretion and insulin action of a 48-h reduction of plasma free fatty acids with acipimox in nondiabetic subjects genetically predisposed to type 2 diabetes. Am J Physiol Endocrinol Metab 2007 Jun; 292(6): E1775–81
Derendorf H, Hochhaus G, Mollmann H, et al. Receptor-based pharmacokinetic-pharmacodynamic analysis of corticosteroids. J Clin Pharmacol 1993 Feb; 33(2): 115–23
Sato S, Katayama K, Kakemi M, et al. A kinetic study of chlorpromazine on the hyperglycemic response in rats: II. Effect of chlorpromazine on plasma glucose. J Pharmacobiodyn 1988 Jul; 11(7): 492–503
Sato S, Mizuno S, Hatanaka T, et al. A kinetic study of chlorpromazine on the hyperglycemic response in rats: I. Effect of chlorpromazine on plasma catecholamines. J Pharmacobiodyn 1988 Jul; 11(7): 486–91
Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia 2000 May; 43(5): 533–49
Lesko LJ, Atkinson Jr AJ. Use of biomarkers and surrogate endpoints in drug development and regulatory decision making: criteria, validation, strategies. Annu Rev Pharmacol Toxicol 2001; 41: 347–66
Colburn WA, Lee JW. Biomarkers, validation and pharmacokinetic-pharmacodynamic modelling. Clin Pharmacokinet 2003; 42(12): 997–1022
Danhof M, Alvan G, Dahl SG, et al. Mechanism-based pharmacokinetic-pharmacodynamic modeling: a new classification of biomarkers. Pharm Res 2005 Sep; 22(9): 1432–7
van Griensven JM, Jusko WJ, Lemkes HH, et al. Tolrestat pharmacokinetic and pharmacodynamic effects on red blood cell sorbitol levels in normal volunteers and in patients with insulin-dependent diabetes. Clin Pharmacol Ther 1995 Dec; 58(6): 631–40
Hamrén B, Ericsson H, Öhman P, et al. Pharmacokinetic and pharmacodynamic modelling of the dual PPAR α/β agonist tesaglitazar in patients with manifestations of insulin resistance [abstract no. 808; online]. Annual Meeting of the Population Approach Group in Europe; 2005 Jun 16–17; Pamplona. Available from URL: http://www.page-meeting.org/default.asp?keuze=search [Accessed 2008 May 14]
van Schaick EA, Zuideveld KP, Tukker HE, et al. Metabolic and cardiovascular effects of the adenosine A1 receptor agonist N6-(p-sulfophenyl)adenosine in diabetic Zucker rats: influence of the disease on the selectivity of action. J Pharmacol Exp Ther 1998 Oct; 287(1): 21–30
Van der Graaf PH, Van Schaick EA, Visser SA, et al. Mechanism-based pharmacokinetic-pharmacodynamic modeling of antilipolytic effects of adenosine A(1) receptor agonists in rats: prediction of tissue-dependent efficacy in vivo. J Pharmacol Exp Ther 1999 Aug; 290(2): 702–9
Jin JY, DuBois DC, Almon RR, et al. Receptor/gene-mediated pharmacodynamic effects of methylprednisolone on phosphoenolpyruvate carboxykinase regulation in rat liver. J Pharmacol Exp Ther 2004 Apr; 309(1): 328–39
Summers RL, Montani J-P. Mathematical model of glucose homeostasis for the study of metabolic states. J Miss Acad Sci 1989; 34: 25–31
Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007 May; 132(6): 2169–80
Rangamani P, Sirovich L. Survival and apoptotic pathways initiated by TNF-alpha: modeling and predictions. Biotechnol Bioeng 2007 Aug 1; 97(5): 1216–29
Keith M, Norwich KH, Wong W, et al. The tissue distribution of tumor necrosis factor-alpha in rats: a compartmental model. Metabolism 2000 Oct; 49(10): 1309–17
Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006 Dec 14; 444(7121): 840–6
Krzyzanski W, Jusko WJ. Note: caution in use of empirical equations for pharmacodynamic indirect response models. J Pharmacokinet Biopharm 1998 Dec; 26(6): 735–41
Black JW, Leff P. Operational models of pharmacological agonism. Proc R Soc Lond B Biol Sci 1983 Dec 22; 220(1219): 141–62
Howard BV, Klimes I, Vasquez B, et al. The antilipolytic action of insulin in obese subjects with resistance to its glucoregulatory action. J Clin Endocrinol Metab 1984 Mar; 58(3): 544–8
Sturm K, Levstik L, Demopoulos VJ, et al. Permeability characteristics of novel aldose reductase inhibitors using rat jejunum in vitro. Eur J Pharm Sci 2006 May; 28(1–2): 128–33
US FDA. Challenge and opportunity on the critical path to new medical products [online]. Rockville (MD): FDA, 2004 Mar. Available from URL: http://www.fda.gov/oc/initiatives/criticalpath/whitepaper.html [Accessed 2008 Apr 10]
Holford NHG, Mould DR, Peck C. Disease progression models. In: Atkinson A, editor. Principles of clinical pharmacology. New York: Academic Press, 2001
Levy J, Atkinson AB, Bell PM, et al. Beta-cell deterioration determines the onset and rate of progression of secondary dietary failure in type 2 diabetes mellitus: the 10-year follow-up of the Belfast Diet Study. Diabet Med 1998 Apr; 15(4): 290–6
Mould DR. Developing models of disease progression. In: Ette EI, Williams PJ, editors. Pharmacometrics. Hoboken (NJ): John Wiley & Sons, Inc., 2007: 547–81
Chan PL, Holford NH. Drug treatment effects on disease progression. Annu Rev Pharmacol Toxicol 2001; 41: 625–59
Post TM, Freijer JI, DeJongh J, et al. Disease system analysis: basic disease progression models in degenerative disease. Pharm Res 2005 Jul; 22(7): 1038–49
Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006 Dec 7; 355(23): 2427–43
Topp B, Promislow K, de Vries G, et al. A model of beta-cell mass, insulin, and glucose kinetics: pathways to diabetes. J Theor Biol 2000 Oct 21; 206(4): 605–19
Hamrén B. Safety and efficacy modelling in anti-diabetic drug development [PhD thesis]. Uppsala: Uppsala University, 2008
Bagust A, Beale S. Deteriorating beta-cell function in type 2 diabetes: a long-term model. QJM 2003 Apr; 96(4): 281–8
Kahn R. Dealing with complexity in clinical diabetes: the value of Archimedes. Diabetes Care 2003 Nov; 26(11): 3168–71
Wilson PW, D’Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation 1998 May 12; 97(18): 1837–47
Brown JB, Russell A, Chan W, et al. The global diabetes model: user friendly version 3.0. Diabetes Res Clin Pract 2000 Nov; 50 Suppl. 3: S15–46
Eddy DM, Schlessinger L. Archimedes: a trial-validated model of diabetes. Diabetes Care 2003 Nov; 26(11): 3093–101
Eddy DM, Schlessinger L. Validation of the Archimedes diabetes model. Diabetes Care 2003 Nov; 26(11): 3102–10
Klinke 2nd DJ. Integrating epidemiological data into a mechanistic model of type 2 diabetes: validating the prevalence of virtual patients. Ann Biomed Eng 2008 Feb; 36(2): 321–34
Young DL, Ramanujan S, Kreuwel HT, et al. Mechanisms mediating anti-CD3 antibody efficacy: insights from a mathematical model of type 1 diabetes. Ann N Y Acad Sci 2006 Oct; 1079: 369–73
Herman WH. Diabetes modeling. Diabetes Care 2003 Nov; 26(11): 3182–3
Schlessinger L, Eddy DM. Archimedes: a new model for simulating health care systems — the mathematical formulation. J Biomed Inform 2002 Feb; 35(1): 37–50
Mount Hood 4 Modeling Group. Computer modeling of diabetes and its complications: a report on the Fourth Mount Hood Challenge Meeting. Diabetes Care 2007 Jun; 30(6): 1638–46
American Diabetes Association Consensus Panel. Guidelines for computer modeling of diabetes and its complications. Diabetes Care 2004 Sep; 27(9): 2262–5
Acknowledgements
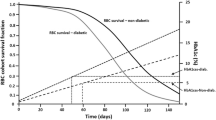
The authors thank Drs Richard Almon and Debra DuBois for their critical review of the manuscript and valuable comments, and Prof. Amanda Almon for preparing the illustrations for figures 1 and 2. This work was supported by the UB-Pfizer Strategic Alliance. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Landersdorfer, C.B., Jusko, W.J. Pharmacokinetic/Pharmacodynamic Modelling in Diabetes Mellitus. Clin Pharmacokinet 47, 417–448 (2008). https://doi.org/10.2165/00003088-200847070-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200847070-00001