Abstract
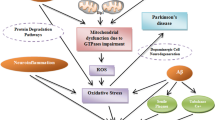
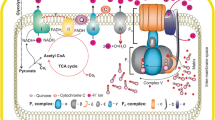
In Alzheimer’s disease (AD) pathogenesis, increasing evidence implicates mitochondrial dysfunction resulting from molecular defects in oxidative phosphorylation (OXPHOS). The objective of the present study was to determine the role of mRNA expression of mitochondrial genes responsible for OXPHOS in brain specimens from early AD and definite AD patients. In the present article, using quantitative real-time polymerase chain reaction (PCR) techniques, we studied mRNA expression of 11 mitochondrial-encoded genes in early AD patients (n=6), definite AD patients (n=6), and control subjects (n=6). Using immunofluorescence techniques, we determined differentially expressed mitochondrial genes—NADH 15-kDa subunit (complex I), cytochrome oxidase subunit 1 (complex IV), and ATPase δ-subunit (complex V)—in the brain sections of AD patients and control subjects. Our quantitative reverse transcription (RT)-PCR analysis revealed a downregulation of mitochondrial genes in complex I of OXPHOS in both early and definite AD brain specimens. Further, the decrease of mRNA fold changes was higher for subunit 1 compared to all other subunits studied, suggesting that subunit 1 is critical for OXPHOS. Contrary to the downregulation of genes in complex I, complexes III and IV showed increased mRNA expressions in the brain specimens of both early and definite AD patients, suggesting a great demand on energy production. Further, mitochondrial gene expression varied greatly across AD patients, suggesting that mitochondrial DNA defects may be responsible for the heterogeneity of the phenotype in AD patients. Our immunofluorescence analyses of cytochrome oxidase and of the ATPase δ-subunit suggest that only subpopulations of neurons are differentially expressed in AD brains. Our double-labeling immunofluorescence analyses of 8-hydroxyguanosine and of cytochrome oxidase suggest that only selective, over-expressed neurons with cytochrome oxidase undergo oxidative damage in AD brains. Based on these results, we propose that an increase in cytochrome oxidase gene expression might be the result of functional compensation by the surviving neurons or an early mitochondrial alteration related to increased oxidative damage.
Similar content being viewed by others
References
Aarskog N. K. and Vedeler C. A. (2000) A new method that detects both the peripheral myelin protein 22 duplication in Charcot-Marie-Tooth type 1A disease and the peripheral myelin protein 22 deletion in hereditary neuropathy with liability to pressure palsies. Hum. Genet. 107, 494–498.
Aksenov M. Y., Tucker H. M., Nair P., et al. (1999) The expression of several mitochondrial and nuclear genes encoding the subunits of electron transport chain enzyme complexes, cytochrome c oxidase, NADH dehydrogenase in different brain regions in Alzheimer’s disease. Neurochem. Res. 24, 767–774.
Aldea C., Alvarez C. P., Folgueira L., Delgado R., and Otero J. R. (2002) Rapid detection of herpes simplex virus DNA in genital ulcers by real-time PCR suing SYBR green I dye as the detection signal. J. Clin. Microbiol. 40, 1060–1062.
Beal M. F. (1998) Mitochondrial dysfunction in neurodegenerative diseases. Biochim. Biophys. Acta 1366, 211–213.
Blass J. P. (1997) Cerebral metabolic impairments. In: Alzheimer’s disease:cause(s), diagnosis, treatment, and care. Khachuaturian Z. S. and Radebaugh T. S. (eds.). CRC Press NY, 1997;187–206.
Blass J. P. (2000) The mitochondrial spiral. An adequate cause of dementia in Alzheimer’s disease. Ann. New Acad. Sci. 924, 170–183.
Blass J. P. (2001) Brain metabolism and brain disease: is metabolic deficiency the proximate cause of Alzheimer dementia? J. Neurosci. Res. 66, 851–856.
Bonilla E., Tanji K., Hirano M., Vu T. H., DiMauro S., and Schon E. A. (1999) Mitochondrial involvement in Alzheimer’s disease. Biochim. Biophys. Acta 1410, 171–182.
Bonilla E., Tanji K., Hirano M., Vu T. H., DiMauuro S., and Schon E. A. (2001) Mitochondrial involvement in Alzheimer’s disease. Biochim. Biophys. Acta: Bioenertics 1410, 171–182.
Braak H. and Braak E. (1991) Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 82, 239–259.
Bustin S. A. (2000) Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 25, 169–193.
Castellano R., Hirai K., Aliev G., et al. (2002) Role of mitochondrial dysfunction in Alzheimer’s disease. J. Neurosci. Res. 70, 357–360.
Cavelier L., Erikson I., Tammi M., et al. (2001) Mt DNA mutations in maternally inherited diabetes: presence of the 3397NDI mutation previously associated with Alzheimer’s and Parkinson’s disease. Hereditas 135, 65–70.
Chandrasekaran K., Giordano T., Brady D. R., et al. (1994) Impairment in gene expression ox oxidative metabolism in vulnerable brain regions in Alzheimer’s disease. Neurobiol. Aging 14, 343–532.
Chandrasekaran K., Hatanpää K., Brady D. R., and Rapoport S. I. (1996) Evidence for physiological down-regulation of brain oxidative phosphorylation in Alzheimer’s disease. Exp. Neurol. 142, 80–88.
Chandrasekaran K., Hatanpää K., Rapoport S. I., and Brady D. R. (1997) Decreased expression of nuclear and mitochdnrial DNA-encoded genes of oxidative phosphorylation in association neocortex in Alzheimer disease. Mol. Brain Res. 44, 99–104.
De la Monte S. M., Luong T. L., Neely T. R., Robinson D., and Wands J. R. (2000) Mitochondrial DNA damage as a mechanism of cell loss in Alzheimer’s disease. Lab. Invest. 80, 1323–1335.
Egensperger R., Kosel S., Schnopp N. M., Mehraein P., and Graeber M. B. (1997) Association of the mitochondrial tRNA (A4336G) mutation with Alzheimer’s and Parkinson’s diseases. Neuropathol. Appl. Neurobiol. 23, 315–321.
Fukuyama R., Hatanpaa K., Rapoport S. I., and Chandrasekaran K. (1996) Gene expression of ND4, a subunit of complex I of oxidative phosphorylation in mitochondria, is deceased in temporal cortex of brains of Alzheimer’s disease patients. Brain Res. 25, 290–293.
Gutala R. V. and Reddy P. H. (2004) The use of real-time PCR analysis in a gene expression study of Alzheimer’s disease postmortem brain. J. Neurosci. Methods 131, 101–107.
Hatanpaa K., Chandrasekaran K., Brady D. R., and Rapoprt S. I. (1998) No association between Alzheimer’s plaques and decreased levels of cytochrome oxidase subunit mRNA, a marker of neuronal energy metabolism. Mol. Brain Res. 59, 13–21.
Hirai K., Aliev G., Nunomura A., et al. (2001) Mitochondrial abnormalities in Alzheimer’s disease. J. Neurosci. 21, 3017–3023.
Hutchin T. P., Heath P. R., Pearson R. C., and Sinclair A. J. (1997) Mitochondrial DNA mutations in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 241, 221–225.
Lin M. T., Simon D. K., Ahn C. H., Kim L. M., and Beal F. M. (2002) High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer’s disease brain. Hum. Mol. Genet. 11, 133–145.
Lin F. H., Lin R., Wisniewski H. M., Hwang Y. W., Grundke-Iqbal I., Healy-Louie G., and Iqbal K. (1992) Detection of point mutations in codon 331 of mitochondrial NADH dehydrogenase subunit 2 in Alzheimer’s brains. Biochem. Biophys. Res. Commun. 182, 238–246.
Mattson M. P., Pederson W. A., Duan W., Culmsee C., and Camandola S. (1999) Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer’s and Parkinson’s diseases. Ann NY Acad. Sci. 893, 154–175.
Ojaimi J. and Byrne E. (2001) Mitochondrial function and Alzheimer’s disease. Bio Signals Recept. 10, 254–262.
Olney R. C., Mougey E. B., Wang J., Shulman D. I., and Sylvester J. E. (2002) Using real-time, quantitative PCR for rapid genotyping of the steroid 21-hydroxylase gene in a north Florida population. J. Clin. Endocrinol. Metab. 87, 735–741.
Orth A. and Schpira A. H. V. (2001) Mitochondria and degenerative diseases. Am. J. Med. Genet. 106, 27–36.
Schapira A. H. (2002) Primary and secondary defects in the mitochondrial respiratory chain. J. Inherit. Meta-Dis. 25, 207–214.
Schapira A. H. V. and Cock H. R. (1999) Mitochondrial myopathies and encepahlomyopathies. Eur. J. Clin. Invest. 29, 886–898.
Shoffner J. M. (1997) Oxidative phosphorylation defects and Alzheimer’s disease. Neurogenetics 1, 13–19.
Schoffner J. M. (2000) Mitochondrial myopathy diagnosis. Neurol. Clin. 18, 105–123.
Shoffner J. M., Brown M. D., Torroni A., et al. (1993) Mitochondrial DNA variants observed in Alzheimer disease and Parkinson disease patients. Genomics 17, 171–184.
Sieber O. M., Lamlum H., Crabtree M. D., et al. (2002) Whole-gene APC deletions cause classical familial adenomatous polyposis, but not attenuated polyposis or “multiple” colorectal adenomas. Proc. Natl. Acad. Sci. USA 99, 2954–2958.
Simonian N. A. and Hyman B. T. (1994) Functional alterations in Alzheimer’s disease: selective loss of mitochondrial-encoded cytochrome oxidase mRNA in the hippocampal formation. J. Neuropathol. Exp. Neurol. 53, 508–512.
Strazielle C., Sturchler-Pierrat C., Staufenbiel M., and Lalonde R. (2003) Regional brain cytochrome oxidase activity in beta-amyloid precursor protein transgenic mice with the Swedish mutation. Neuroscience 118, 1151–1163.
User Bulletin no. 2 (1997) ABI Prism 7700 Sequence Detection System.
Wallace D. C. (1999) Mitochondrial diseases in man and mouse. Science 283, 1482–1488.
Wallace D. C., Lott M. T., and Brown M. D. (1997) Mitochondrial defects in neurodegenerative diseases and aging. In Mitochondria and Free Radicals in Neurodegenerative Diseases. Beal F., Howell N., and Bodis-Wollner I., eds.). Wiley-Liss: NY, pp. 283–308.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manczak, M., Park, B.S., Jung, Y. et al. Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease. Neuromol Med 5, 147–162 (2004). https://doi.org/10.1385/NMM:5:2:147
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/NMM:5:2:147