-
PDF
- Split View
-
Views
-
Cite
Cite
Shanshan Cai, Katsumasa Sato, Toshiaki Shimizu, Seiko Yamabe, Miho Hiraki, Chiaki Sano, Haruaki Tomioka, Antimicrobial activity of picolinic acid against extracellular and intracellular Mycobacterium avium complex and its combined activity with clarithromycin, rifampicin and fluoroquinolones, Journal of Antimicrobial Chemotherapy, Volume 57, Issue 1, January 2006, Pages 85–93, https://doi.org/10.1093/jac/dki418
Close - Share Icon Share
Abstract
Objectives: A natural metal ion chelator, picolinic acid (PA), is known to potentiate macrophage antimycobacterial activity. Here, we studied the antimicrobial activity of PA against extracellular and intramacrophage Mycobacterium avium complex (MAC) organisms.
Methods: MAC organisms, MAC-infected macrophages or MAC-infected type II pneumocytes were cultured in the presence or absence of PA with or without antimycobacterial drugs, and residual bacterial cfu of extracellular or intracellular MAC were counted on 7H11 agar plates.
Results: First, PA exhibited antimicrobial activity against extracellular and intramacrophage MAC. The effect of PA was mimicked by other metal ion-chelating agents, such as ethylenediamine tetraacetic acid and O,O′-bis (2-aminophenyl) ethyleneglycol-N,N,N′,N′-tetraacetic acid. Second, PA potentiated antimicrobial effects of a two-drug combination of clarithromycin/rifampicin and some fluoroquinolones (levofloxacin, sitafloxacin and gatifloxacin) against extracellular and intramacrophage MAC. Similar combined effects of PA with clarithromycin/rifampicin were also seen in the case of MAC residing within type II alveolar epithelial cells.
Conclusions: PA exerted an appreciable anti-MAC activity, when used singly or in combination with some antimycobacterial drugs (clarithromycin/rifampicin and fluoroquinolones), suggesting the usefulness of PA as an adjunct for clinical antimicrobial chemotherapy of MAC infections.
Introduction
Clinical management of Mycobacterium avium complex (MAC) infection is difficult, since it is frequently encountered in immunocompromised hosts, particularly AIDS patients, and MAC organisms are moderately to highly resistant to common antituberculosis drugs such as isoniazid, ethambutol, pyrazinamide and rifampicin.1 Some macrolides, such as clarithromycin and azithromycin, in combination with rifamycin derivatives (rifampicin and rifabutin) or other drugs are appreciably efficacious in treating disseminated MAC infections in AIDS patients. Despite this, MAC pulmonary infections are generally intractable owing to a lack of anti-MAC therapeutics (and their companion drugs) with excellent in vivo activity.1–4 The urgent problems are that therapeutic efficacies of present multidrug regimens of patients with MAC infection, in terms of sputum conversion and clinical improvement of patients and the relapse rate after combined chemotherapy, are at unsatisfactory levels.5,6 Since the development of new classes of anti-MAC drugs is unpromising at present, the most practical strategy is to develop regimens to treat patients with refractory MAC diseases using ordinary antimycobacterial drugs in combination with adjunctive immunomodulators (called adjunctive immunotherapy).7
For this purpose, development of new classes of immunomodulators other than immunopotentiating cytokines [interferon-γ (IFN-γ), interleukin-12, etc.], particularly those with no severe adverse effects and low cost, is desired. Such types of immune response modifiers, which appear to be useful in potentiating host resistance to mycobacterial infections, include ATP and its analogues, imidazoquinoline, diethyldithiocarbamate, poloxamer, dibenzopyran, 1α,25-dihydroxyvitamin D3, glucosaminyl muramyl dipeptide, and heat-killed Mycobacterium vaccae.7
Picolinic acid (PA), a naturally occurring product of degradation of tryptophan, is a cheap and safe chelating agent for metal ions, such as Zn2+ and Fe2+ ions, and chromium picolinate is generally used by overweight or obese persons as a dietary supplement that may promote weight loss.8,9 PA is also known to up-regulate host immune responses, especially macrophage cell functions.10,11 It has recently been demonstrated by Pais and Appelberg12 that PA reduced intramacrophage growth of MAC organisms. Notably, PA in combination with IFN-γ completely inhibits mycobacterial growth in macrophages, and this effect of PA is dependent on host macrophage apoptosis but independent of macrophage activity in producing reactive nitrogen intermediates (RNIs), reactive oxygen intermediates (ROIs) and tumour necrosis factor-α (TNF-α).12 In this context, it has also been reported that PA is endowed with a regulatory effect on the cell cycle and inhibitory effects on bacterial growth and tumour cell growth.13–15 Therefore, it is possible that PA-mediated intracellular bacterial killing in MAC-infected macrophages12 may be in part owing to antimicrobial activity of PA against MAC organisms. In this study, we thus examined the antimicrobial activity of PA against extracellular MAC and found that it actually exerted significant levels of growth inhibitory activity against MAC. We also studied profiles of the antimicrobial activity of PA against intracellular MAC residing within macrophages (professional phagocytes) and type II pneumocytes (non-professional phagocytes), in particular, in terms of combined effects of PA with antimycobacterial drugs, including clarithromycin, rifampicin and fluoroquinolones.
Materials and methods
Microorganisms
MAC N-444 (serovar 8) and MAC N-260 (serovar 16), which were isolated from patients with MAC infection in Japan and identified as M. avium and Mycobacterium intracellulare, respectively, by DNA probe testing, were used. For preparation of a bacterial suspension as an inoculum, test organisms were grown in 7H9 broth (Difco Laboratories, Detroit, MI, USA) at 37°C. Bacterial suspensions prepared with PBS containing 1% BSA were gently sonicated using a sonicator (Model UR-20P; Tomy Seiko Co., Tokyo, Japan) for 5 s and centrifuged at 150 g for 5 min to eliminate bacterial clumps, and the upper layer (about 80% volume) was saved as an inoculum. The bacterial suspension prepared in this fashion contained no obvious bacterial clumps on microscopic observation.
Mice
Female BALB/c mice (8–12 weeks old) purchased from Japan Clea Co., Osaka, Japan were used. The animal studies performed in the present paper were given the ethics committee approval of Shimane University School of Medicine.
Special agents
Special agents and antimicrobial drugs used in this study were as follows: clarithromycin (Taisho Pharmaceutical Co., Tokyo, Japan), rifampicin (Wako Pure Chemical Industries, Osaka, Japan), levofloxacin (Daiichi Pharmaceutical Co., Tokyo, Japan), sitafloxacin (Daiichi Pharmaceutical Co.), gatifloxacin (Kyorin Pharmaceutical Co., Tokyo, Japan), PA (Sigma, St Louis, MO, USA), N-(2-hydroxyethyl) iminodiacetic acid (HIDA) (Dojindo Laboratories, Kumanoto, Japan), ethylendiamine-N,N,N′,N′-tetraacetic acid (EDTA) (Dojindo), O,O′-bis(2-aminophenyl) ethyleneglycol-N,N,N′,N′-tetraacetic acid (BAPTA) (Dojindo), phorbol 12-myristate 13-acetate (PMA) (RBI Ltd, Natick, MA, USA), fetal bovine serum (FBS) (Bio Whittaker Co., Walkersville, MD, USA) and zymosan A (Sigma).
MIC determination
MICs of test antimicrobial drugs and PA for the test organisms (MAC N-444 and MAC N-260) were determined by the broth dilution method using 7HSF medium as previously reported.16
Antimicrobial activity of PA against extracellular MAC
Test MAC organisms (5 × 104 or 1 × 106 cfu) were cultured in 1 mL of 7HSF medium (a broth medium with the same composition as Middlebrook 7H11 agar but without Malachite Green) with or without the addition of PA at 37°C in an incubator (100% humidified air) for up to 7 days. At intervals, the incubation mixture was centrifuged at 2000 g for 20 min and the recovered microorganisms were then washed with distilled water by centrifugation. The resulting bacterial pellet was suspended in 0.25 mL of distilled water and the number of cfu of recovered organisms was counted on 7H11 agar plates.
Intracellular growth of MAC
The intracellular growth of MAC organisms inside mouse peritoneal macrophages, THP-1 human macrophages (ATCC, Rockville, MD, USA), and A-549 human type II alveolar epithelial cells (A-549 cells) (ATCC) was measured by the following experimental systems.16,17
Mouse macrophage system
Zymosan A-induced murine peritoneal exudate cells (3 × 105), suspended in 0.2 mL of 5% FBS-RPMI 1640 medium (RPMI medium), were seeded in a microculture well (96-well flat-bottom plate; Becton Dickinson & Company, Lincoln Park, NJ, USA) and incubated at 37°C in a CO2 incubator (5% CO2/95% humidified air) for 2 h. After washing with 2% FBS-Hanks' balanced salt solution (HBSS), the resultant macrophage monolayer was infected with test MAC organisms by incubating it in the medium (100 μL) containing 6 × 106 cfu of bacteria in a CO2 incubator for 1.5 h. Infected macrophages were then washed with 2% FBS-HBSS and then cultivated in 0.2 mL of 5% FBS-RPMI medium in the presence or the absence of test drugs in a CO2 incubator for up to 7 days. At intervals cultured macrophages were lysed with 0.07% SDS for 10 min. After neutralization of SDS with 6% BSA, the resultant macrophage cell lysate was centrifuged at 2000 g for 20 min to collect bacterial cells and the number of cfu of recovered organisms was counted on 7H11 agar plates.
THP-1 macrophage system
THP-1 monocytic cells (5 × 104 cells) were cultured in 5% FBS-RPMI medium containing 50 ng/mL PMA in a microculture well at 37°C in a CO2 incubator for 18–22 h, in order to differentiate the monocytic cells to mature macrophages. After washing with 2% FBS-HBSS, the resultant macrophage monolayer was infected with test MAC organisms by incubating it in the medium (0.1 mL) containing 1 × 106 cfu of bacteria in a CO2 incubator for 1.5 h. After rinsing with 2% FBS-HBSS, infected macrophages were cultured in 0.2 mL of 5% FBS-RPMI medium in the presence or the absence of test drugs in a CO2 incubator for up to 7 days. At intervals the number of residual bacterial cfu in cultured macrophages was counted as mentioned for the mouse macrophage system.
A-549 cell system
A-549 cells (4 × 104 cells) suspended in 5% FBS-RPMI medium were seeded in 96-well tissue culture plates and incubated for 18 h in order to allow attachment to the wells. After washing with 2% FBS-HBSS, the A-549 cells were infected with MAC (8 × 105 cfu) in a 100 μL portion of the same medium for 2 h. After rinsing with 2% FBS-HBSS, the infected cells were cultured in 200 μL of 1% FBS-RPMI medium in the presence or absence of test drugs for up to 7 days. At intervals, viable count analysis was done as mentioned above.
Statistical analysis
Statistical analysis was performed by Bonferroni's multiple t-test using StatView software (Hulinks, Inc., Tokyo, Japan).
Results
Antimicrobial activity of PA against extracellular MAC
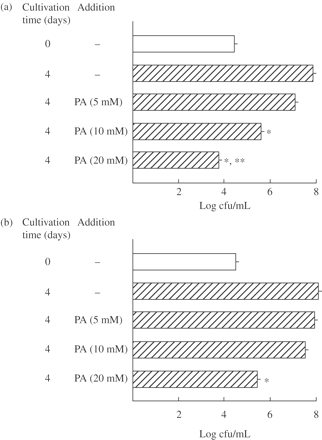
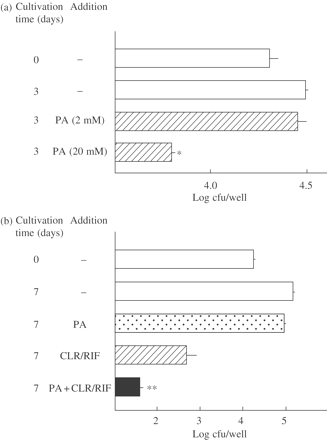
PA was examined for its antimicrobial activity against extracellular MAC N-444 strain (low-virulence MAC)18 and N-260 strain (high-virulence MAC).18 As shown in Figure 1, PA caused growth inhibition of both MAC strains in a dose-dependent manner. PA at 20 mM completely inhibited bacterial growth of MAC N-444 and, moreover, exerted a weak bactericidal effect (Figure 1a). However, as indicated in Figure 1(b), the antimicrobial activity of PA against MAC N-260 was significantly weaker than its activity against MAC N-444 (P < 0.01). PA even at 20 mM caused only partial but significant growth inhibition of MAC N-260. Table 1 shows MICs (in terms of μM and mg/L concentrations) of PA, clarithromycin, rifampicin and fluoroquinolones for MAC N-444 and N-260 strains measured by the broth dilution method using 7HSF medium. The MIC of PA for MAC N-444 was smaller than that for MAC N-260, confirming that the low-virulence MAC N-444 strain is more susceptible to PA compared with the high-virulence MAC N-260 strain. Although the MICs of PA for the MAC strains were high (25–50 mM), these concentrations of PA are known not to cause severe cytotoxic effects, as described in the Discussion section.
Antimicrobial activity of PA against extracellular MAC. MAC N-444 (a) and N-260 (b) strains were cultured in 7HSF medium with or without addition of PA at indicated concentrations at 37°C for 4 days and then bacterial cfu counting was done on 7H11 agar plates. Each bar indicates the mean ± SEM (n = 3). *Significantly smaller than the control value (none added) (P < 0.01). **Significant bactericidal activity (P < 0.01).
MICs of test antimicrobials and PA for MAC N-444 and MAC N-260 strains
| . | MIC (μM) . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain . | CLR . | RIF . | LVX . | STX . | GAT . | PA . | |||||
| N-444 | 5.4 (4) | 9.7 (8) | 22 (16) | 4.9 (2) | 11 (4) | 25 × 103 (3 × 103) | |||||
| N-260 | 5.4 (4) | 9.7 (8) | 22 (16) | 9.8 (4) | 21 (8) | 50 × 103 (6 × 103) | |||||
| . | MIC (μM) . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain . | CLR . | RIF . | LVX . | STX . | GAT . | PA . | |||||
| N-444 | 5.4 (4) | 9.7 (8) | 22 (16) | 4.9 (2) | 11 (4) | 25 × 103 (3 × 103) | |||||
| N-260 | 5.4 (4) | 9.7 (8) | 22 (16) | 9.8 (4) | 21 (8) | 50 × 103 (6 × 103) | |||||
CLR, clarithromycin; RIF, rifampicin; LVX, levofloxacin; STX, sitafloxacin; GAT, gatifloxacin.
In parentheses, MICs are indicated in terms of mg/L.
MICs of test antimicrobials and PA for MAC N-444 and MAC N-260 strains
| . | MIC (μM) . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain . | CLR . | RIF . | LVX . | STX . | GAT . | PA . | |||||
| N-444 | 5.4 (4) | 9.7 (8) | 22 (16) | 4.9 (2) | 11 (4) | 25 × 103 (3 × 103) | |||||
| N-260 | 5.4 (4) | 9.7 (8) | 22 (16) | 9.8 (4) | 21 (8) | 50 × 103 (6 × 103) | |||||
| . | MIC (μM) . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain . | CLR . | RIF . | LVX . | STX . | GAT . | PA . | |||||
| N-444 | 5.4 (4) | 9.7 (8) | 22 (16) | 4.9 (2) | 11 (4) | 25 × 103 (3 × 103) | |||||
| N-260 | 5.4 (4) | 9.7 (8) | 22 (16) | 9.8 (4) | 21 (8) | 50 × 103 (6 × 103) | |||||
CLR, clarithromycin; RIF, rifampicin; LVX, levofloxacin; STX, sitafloxacin; GAT, gatifloxacin.
In parentheses, MICs are indicated in terms of mg/L.
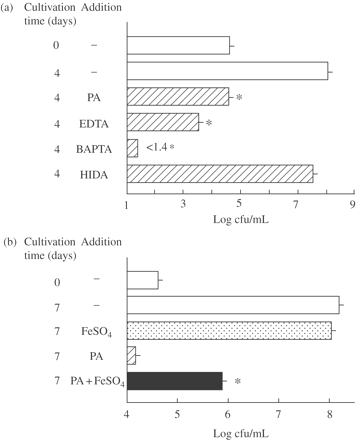
Since PA chelates metal ions, such as Zn2+ and Fe2+, it is likely that its antimicrobial activity against MAC organisms is owing to its ability to chelate metal ions, especially Fe ions which are an essential nutrient for bacteria. As shown in Figure 2(a), not only PA but also other kinds of metal ion chelators, including EDTA (a chelator for Ca2+, Mg2+, Fe2+, etc.) and BAPTA (a chelator for Fe2+, Fe3+ and Ca2+), had antimicrobial activity against extracellular MAC N-444 and HIDA (a Fe2+-specific iron chelator) exerted a weak anti-MAC action. Notably both EDTA and BAPTA exerted bactericidal activity against MAC. A chelator for Fe2+, Fe3+ and Ca2+ ions, BAPTA, exhibited the most potent inhibitory action. This is consistent with previous finding by Gomes et al.19 that some iron chelating agents, such as desferrioxamine and N,N′bis(2-hydroxybenzyl) ethylenediamine-N,N′-diacetic acid were active in inhibiting extracellular and intramacrophage growth of MAC. Moreover, the addition of 2 mM FeSO4 to 7HSF medium caused partial but significant restoration of PA-mediated inhibition of extracellular MAC N-444 (Figure 2b). Therefore, it appears that the activity of PA in inhibiting/killing extracellular MAC is principally attributable to its iron-chelating function, which causes the depletion of nutrient iron essential for bacterial growth. These findings indicate that the potentiation of anti-MAC antimicrobial function of macrophages by treatment with PA previously observed by Pais and Appelberg12 is at least partly owing to the direct antimicrobial action of PA against MAC organisms through depriving the bacteria of available nutrient iron.
Antimicrobial activities of PA and other metal ion-chelating agents against extracellular MAC (a) and inhibitory effects of FeSO4 against the anti-MAC antimicrobial activity of PA (b). (a) MAC N-444 was cultured in 7HSF medium with or without addition of PA, EDTA, BAPTA or HIDA at 20 mM each for 4 days. *Significant inhibitory activity (P < 0.01). (b) MAC N-444 was cultured in 7HSF medium in the presence or absence of PA (20 mM) and/or FeSO4 (2 mM) for 7 days. *Significant blocking activity (P < 0.01). The other details are the same as in Figure 1.
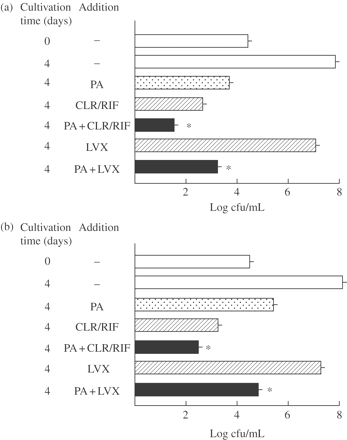
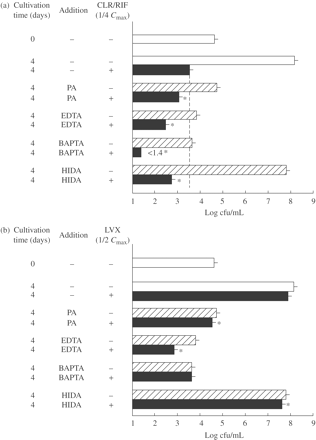
As shown in Figure 3, PA in combination with clarithromycin/rifampicin exhibited significant combined effects against extracellular MAC N-444 and N-260 strains. However, moderate combined effects were achieved by using PA in combination with levofloxacin in both the cases of MAC N-444 and N-260 strains. Figure 4 shows the antimicrobial effects of PA and other Fe-chelating agents (EDTA, BAPTA and HIDA) in combination with antimycobacterial drugs (clarithromycin/rifampicin, levofloxacin) against extracellular MAC N-444. All the metal ion-chelating agents exhibited significant combined effects with clarithromycin/rifampicin (Figure 4a). Notably, the most obvious combined effect was seen between BAPTA and clarithromycin/rifampicin, followed by EDTA and clarithromycin/rifampicin. This finding indicates that all the test agents with Fe-chelating ability (PA, EDTA, BAPTA and HIDA) are capable of exerting potently increased antimicrobial activity against extracellular MAC organisms when used in combination with clarithromycin/rifampicin. However, somewhat weak but significant levels of combined effects were seen for combinations of levofloxacin with the test chelating agents (Figure 4b). In this experiment, the most obvious combined effect was seen between EDTA and levofloxacin, but BAPTA showed no combined effect when used with levofloxacin. Notably, in most cases, test chelating agents having Fe-chelating ability potentiated the anti-MAC antimicrobial activities of clarithromycin/rifampicin and levofloxacin when used in combination with the antimycobacterial drugs.
Antimicrobial activity of PA, used singly or in combination with some antimycobacterial drugs, against extracellular MAC. MAC N-444 (a) and N-260 (b) strains were cultured in 7HSF medium with or without addition of 20 mM PA, clarithromycin (CLR)/rifampicin (RIF) (1/4 Cmax each: CLR, 0.58 mg/L; RIF, 1.6 mg/L) or levofloxacin (LVX) (1/2 Cmax, 1.0 mg/L) alone or in combination for 4 days. *Significant combined effect (P < 0.01). The other details are the same as in Figure 1.
Antimicrobial activity of PA and other metal ion-chelating agents, used singly or in combination with clarithromycin/rifampicin (a) or levofloxacin (b), against extracellular MAC. MAC N-444 strain was cultured in 7HSF medium with or without addition of 20 mM PA or 10 mM of other metal ion-chelating agents (EDTA, BAPTA, HIDA), clarithromycin (CLR)/rifampicin (RIF) (1/4 Cmax each: CLR, 0.58 mg/L; RIF, 1.6 mg/L) or levofloxacin (LVX) (1/2 Cmax, 1.0 mg/L) alone or in combination for 4 days. *Significant combined effect (P < 0.01). The other details are the same as in Figure 1.
Antimicrobial activity of PA, used singly or in combination with some antimycobacterial drugs, against intramacrophage MAC
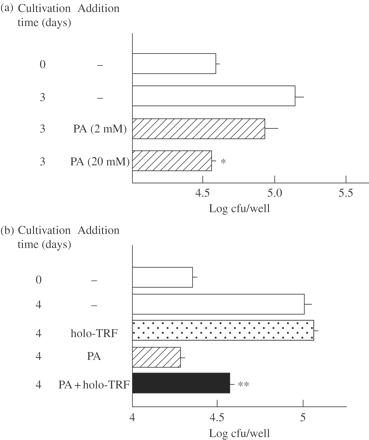
We next examined the antimicrobial activity of PA and its effects on the expression of antimicrobial activities of some antimycobacterial drugs against intramacrophage MAC. As shown in Figure 5(a), PA caused growth inhibition of MAC N-444 residing in THP-1 human macrophages in a dose-dependent manner. Notably, as shown in Figure 5(b), PA activity against intramacrophage MAC N-444 was partly blocked by the addition of holo-transferrin (iron-saturated transferrin) at 1 mg/mL concentration. In separate experiments, apo-transferrin at 1 mg/mL inhibited intramacrophage growth of MAC N-444 by 58 ± 1% (n = 4) and exhibited significant combined effects with PA against the intramacrophage MAC organisms (P < 0.01): PA alone, 76 ± 1% inhibition; PA + apo-transferrin, 86 ± 2% (H. Tomioka, S. Cai, K. Sato and T. Shimizu, unpublished results). It thus appears that holo-transferrin, added in culture medium, is efficiently delivered to and accumulated in macrophage phagosomal vesicles engulfing mycobacterial organisms, as reported by Clemens and Horwitz,20 and subsequently supplies sufficient amounts of essential nutrient iron to MAC organisms replicating inside macrophage phagosomal vesicles. In contrast, it is thought that apo-transferrin molecules delivered to macrophage phagosomal vesicles cause moderate levels of iron deprivation for intraphagosomal MAC organisms and that PA further potentiates this effect.
Antimicrobial activity of PA against intramacrophage MAC (a) and blocking effects of holo-transferrin (holo-TRF) on the antimicrobial activity of PA against intramacrophage MAC (b). (a) THP-1 macrophages infected with MAC N-444 were cultivated in 5% FBS-RPMI medium with or without addition of 2 or 20 mM PA at 37°C in a CO2 incubator for 3 days. (b) MAC N-444-infected THP-1 macrophages were cultured in the medium in the presence or absence of 20 mM PA or holo-TRF (1 mg/mL) or in combination for 4 days. After cultivation, residual intracellular bacterial cfu was counted. *Significantly smaller than the control value (P < 0.01). **Significant blocking effects (P < 0.01). The other details are the same as in Figure 1.
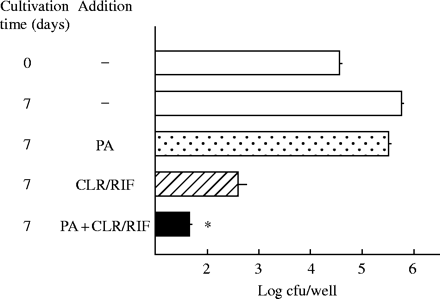
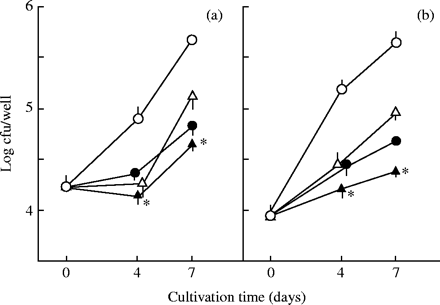
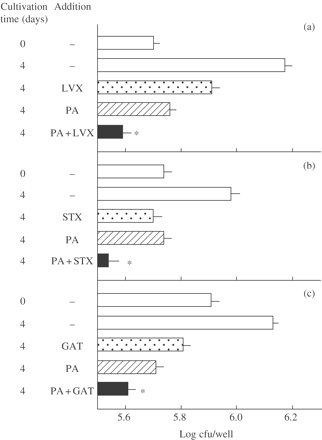
Next, we examined the antimicrobial activity of PA against intramacrophage MAC, when used in combination with some antimycobacterial drugs at concentrations achievable by oral administration of them at clinical dosages. As illustrated in Figure 6, PA potentiated the bactericidal activity of clarithromycin/rifampicin at Cmax concentrations against intramacrophage MAC. Next, we examined the effects of PA on the expression of antimicrobial activities of some fluoroquinolones, added at Cmax concentrations, against intramacrophage MAC. As shown in Figure 7, PA significantly increased the growth inhibitory activity of levofloxacin and sitafloxacin against intracellular MAC replicating within THP-1 macrophages, although fluoroquinolones are generally known to be weakly efficacious in chemotherapy of MAC infections in humans and in animals.3,21–23 In this case, these quinolones in combination with PA failed to exhibit microbicidal effects on MAC organisms replicating within THP-1 macrophages. As shown in Figure 8, similar results were also obtained when mouse peritoneal macrophages were used instead of THP-1 macrophages. Significant combined effects were observed between PA and levofloxacin, sitafloxacin and gatifloxacin. Notably, these quinolones were more or less endowed with bactericidal activity against intramacrophage MAC by combination with PA.
PA-mediated potentiation of antimicrobial effects of clarithromycin (CLR)/rifampicin (RIF) against intramacrophage MAC. MAC N-444-infected THP-1 macrophages were cultured in the medium with or without addition of PA (20 mM) or CLR/RIF (Cmax: CLR, 2.3 mg/L; RIF, 6.2 mg/L) alone or in combination for 7 days. *Significant combined effects (P < 0.01). The other details are the same as in Figure 5.
PA-mediated potentiation of antimicrobial effects of levofloxacin (a) and sitafloxacin (b) against intramacrophage MAC. MAC N-444-infected THP-1 macrophages were cultured in the medium with or without addition of PA (20 mM) or test quinolone (Cmax: levofloxacin, 2.0 mg/L; sitafloxacin, 0.51 mg/L) alone or in combination for up to 7 days. Open circles, none added; open triangles, test quinolone; filled circles, PA (20 mM); filled triangles, test quinolone + PA. *Significant combined effects (P < 0.05). The other details are the same as in Figure 5.
PA-mediated potentiation of antimicrobial effects of some fluoroquinolones against intracellular MAC replicating within mouse peritoneal macrophages. MAC N-444-infected macrophages were cultivated in the medium with or without addition of PA (20 mM) or levofloxacin (LVX) (a), sitafloxacin (STX) (b) or gatifloxacin (GAT) (c) (Cmax: LVX, 2.0 mg/L; STX, 0.51 mg/L; GAT, 1.6 mg/L) alone or in combination for 4 days. *Significant combined effects (P < 0.05). The other details are the same as in Figure 5.
Antimicrobial activity of PA, used singly or in combination with clarithromycin/rifampicin, against intracellular MAC residing inside type II pneumocytes
It is known that type II alveolar epithelial cells play roles as sites of bacterial entry and growth in lung infections owing to mycobacterial pathogens.21,24,25 Thus, we examined the antimicrobial activity of PA and its combined effects with clarithromycin/rifampicin against intracellular MAC residing within A-549 cells. As shown in Figure 9(a), PA at 20 mM concentration exhibited bactericidal activity against MAC residing inside A-549 cells. Moreover, PA significantly potentiated the antimicrobial activity of Cmax concentrations of clarithromycin/rifampicin against intracellular MAC replicating within A-549 cells (Figure 9b). Since A-549 cells are lacking in effective antimicrobial functions against MAC organisms,21,26 it appears that the observed activity of PA against intracellular MAC inside A-549 cells is mainly owing to the direct antimicrobial activity of PA itself.
Antimicrobial activity of PA, used singly (a) or in combination with clarithromycin (CLR)/rifampicin (RIF) (b), against intracellular MAC residing within A-549 cells. (a) MAC N-444-infected A-549 cells in 1% FBS-RPMI 1640 medium with or without addition of 2 or 20 mM PA for 3 days. (b) MAC-infected A-549 cells were cultured in the medium with or without PA (20 mM) or CLR/RIF (Cmax: CLR, 2.3 mg/L; RIF, 6.2 mg/L) alone or in combination for 7 days. *Significantly smaller than the control value (P < 0.01). **Significant combined effects (P < 0.01). The other details are the same as in Figure 5.
Discussion
In the present study, it was found that PA exhibited direct antimicrobial activity against extracellular and intramacrophage MAC organisms presumably by depriving intraphagosomal microenvironment of free nutrient iron essential for the intraphagosomal growth of the parasites (Figures 2a, b and 5b). This is consistent with the finding by Pais and Appelberg12 that the mechanisms of PA-mediated augmentation of macrophage anti-MAC activity do not involve ROIs and RNIs that are known as the major antimicrobial effector molecules produced by macrophages.27,28 It thus appears that the activity of PA in potentiating macrophage anti-MAC activity is largely attributable to its direct antimicrobial activity against MAC organisms. In this context, it has been reported that an excess of iron hampers the Nramp-1-encoded function of host macrophages in exerting antimicrobial activity against intraphagosomal MAC organisms, indicating the function of Nramp-1-encoded protein in the transport of iron out of the parasite-harbouring macrophage phagosomes.29
As indicated in Figure 8(a), PA exhibited significant combined activity against intramacrophage MAC when used in combination with levofloxacin as well as two other quinolones (sitafloxacin and gatifloxacin). In contrast, as shown in Figure 4(b), PA exerted only weak combined activity against extracellular MAC when used in combination with levofloxacin. However, PA displayed strong combined effects against extracellular as well as intramacrophage MAC when used in combination with clarithromycin/rifampicin (Figures 4a and 6). These findings indicate that the mechanisms, by which PA potentiates the antimicrobial activities of clarithromycin/rifampicin and levofloxacin against intramacrophage MAC, may be essentially different from each other. That is, the antimicrobial action of PA owing to its Fe-chelating activity appears to play central roles in the potentiation of the antimicrobial activity of clarithromycin/rifampicin against intramacrophage MAC. On the contrary, the macrophage-activating effects of PA seem to play the major role in the augmentation of the antimicrobial activity of levofloxacin against MAC residing within macrophages.
In this context, recent studies have suggested a role for tryptophan catabolite PA as an important regulator of macrophage activities. It has been reported that PA is a selective inducer of macrophage inflammatory protein-1α and -1β.30 Moreover, it has been indicated that PA exhibited protective effects on mice infected with Candida albicans, by increasing the expression of TNF-α and interleukin-1 at sites of infection, presumably owing to activation of host macrophages.31 It thus appears that PA may potentiate macrophage antimicrobial activity by up-regulating macrophage production of antimicrobial effector molecules other than ROIs and RNIs. In such macrophage antimicrobial functions, participation of free fatty acids (such as arachidonic acid) and cationic antimicrobial peptides (such as α,β-defensins and protegrin-like peptides)32–35 seems to be critical.
Since the development of new classes of anti-MAC drugs is unpromising at present, the most practical strategy is to develop adjunctive drugs for current chemotherapeutic regimens.7 In this context, PA is a cheap and safe metal ion-chelating agent and facilitates absorption of Zn2+ and chromium ions from the intestine when orally administered, and chromium picolinate is generally used by overweight or obese persons as a dietary supplement for weight loss and muscle building.8,9 It has been reported that intraperitoneal administration of PA to rats at a dose of 30 mmol (presumably yielding ∼200–400 mM concentrations in the body fluids) caused no marked histological and ultrastructural changes in the brain.36 This may mean that the doses of PA (∼20 mM) used here for macrophage studies do not cause severe toxic effects in vivo.
In the present study, it was found that PA significantly potentiated the antimicrobial activities of clarithromycin/rifampicin and fluoroquinolones (levofloxacin, sitafloxacin and gatifloxacin) against intramacrophage MAC when used in combination with these antimicrobials. This finding suggests that PA may be useful as an adjunct of antimicrobial chemotherapy of MAC patients with antimycobacterial drugs. It is of interest to note that PA exerted significant levels of combined activity against intracellular MAC residing inside macrophages and lung epithelial cells when added to the medium in combination with fluoroquinolones. This finding suggests possible usefulness of fluoroquinolones for chemotherapy of MAC infections when administered in combination with PA as an adjunctive agent. This is meaningful from the viewpoint of chemotherapy of MAC diseases, because fluoroquinolones have only weak activity against MAC organisms, have no significant clinical efficacy when used as monotherapy, and are generally thought not to significantly serve as companion drugs for the standard anti-MAC multidrug regimens,21–23,37 although it has recently been reported that moxifloxacin is efficacious in treating MAC-infected mice.38
In any case, the present study gave us the following important and promising findings: PA potentiates antimicrobial activities of clarithromycin/rifampicin and fluoroquinolones against both extracellular MAC and intracellular MAC residing within macrophages and lung epithelial cells. Further studies are currently under way using in vivo experimental systems.
Transparency declarations
No declarations were made by the authors of this paper.
We thank Taisho Pharmaceutical Co., Daiichi Pharmaceutical Co. and Kyorin Co. for providing clarithromycin and test fluoroquinolones. This study was supported in part by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
Inderlied CB, Kemper CA, Bermudez LE. The Mycobacterium avium complex.
Benson CA. Disseminated Mycobacterium avium complex infection: implications of recent clinical trials on prophylaxis and treatment.
Tomioka H. Prospects for development of new antimycobacterial drugs.
Benson CA, Williams PL, Currier JS et al. A prospective, randomized trial examining the efficacy and safety of clarithromycin in combination with ethambutol, rifabutin, or both for the treatment of disseminated Mycobacterium avium complex disease in persons with acquired immunodeficiency syndrome.
American Thoracic Society. Diagnosis and treatment of disease caused by nontuberculous mycobacteria.
Kobashi Y, Okimoto N, Matsushima T et al. Effect of combined chemotherapy following the guidelines on treatment for Mycobacterium avium complex pulmonary disease.
Tomioka H. Adjunctive immunotherapy of mycobacterial infections.
Evans GW, Johnson PE. Characterization and quantitation of a zinc-binding ligand in human milk.
Vincent JB. The potential value and toxicity of chromium picolinate as a nutritional supplement, weight loss agent and muscle development agent.
Ruffmann R, Welker RD, Saito T et al. In vivo activation of macrophages but not natural killer cells by picolinic acid (PLA).
Varesio L, Clayton M, Blasi E et al. Picolinic acid, a catabolite of tryptophan, as the second signal in the activation of IFN-gamma-primed macrophages.
Pais TF, Appelberg R. Macrophage control of mycobacterial growth induced by picolinic acid is dependent on host cell apoptosis.
Johnson GS, Fernandez-Pol JA. NRK cells synchronized in G1 by picolinic acid are super-sensitive to prostaglandin E1 stimulation.
Collins JJ, Alder CR, Fernandez-Pol JA et al. Transient growth inhibition of Escherichia coli K-12 by ion chelators: “in vivo” inhibition of ribonucleic acid synthesis.
Leuthauser SW, Oberley LW, Oberley TD. Antitumor activity of picolinic acid in CBA/J mice.
Sato K, Akaki T, Tomioka H. Antimicrobial activities of benzoxazinorifamycin KRM-1648, clarithromycin and levofloxacin against intracellular Mycobacterium avium complex phagocytosed by murine peritoneal macrophages.
Sato K, Tomioka H. Antimicrobial activities of benzoxazinorifamycin (KRM-1648) and clarithromycin against Mycobacterium avium-intracellulare complex within murine peritoneal macrophages, human macrophage-like cells and human alveolar epithelial cells.
Tomioka H, Saito H, Sato K et al. Comparison of the virulence for mice of Mycobacterium avium and Mycobacterium intracellulare identified by DNA probe test.
Gomes MS, Dom G, Pedrosa J et al. Effects of iron deprivation on Mycobacterium avium growth.
Clemens DL, Horwitz MA. The Mycobacterium tuberculosis phagosome interacts with early endosomes and is accessible to exogenously administered transferrin.
Tomioka H. Type II pneumocytes in the evaluation of drug antimycobacterial activity.
Jacobs MR. Fluoroquinolones as chemotherapeutics against mycobacterial infections.
Tomioka H. Present status and future prospects of chemotherapeutics for intractable infections due to Mycobacterium avium complex.
Bermudez LE, Goodman J. Mycobacterium tuberculosis invades and replicates within type II alveolar cells.
Sato K, Tomioka H, Shimizu T et al. Type II alveolar cells play roles in macrophage-mediated host innate resistance to pulmonary mycobacterial infections by producing proinflammatory cytokines.
Sato K, Ogasawara K, Tomioka H et al. Internalization and replication of Mycobacterium tuberculosis and M. avium complex within type II alveolar epithelial cell line.
Chan I, Kaufmann SHE. Immune mechanisms of protection. In: Bloom BR, ed. Tuberculosis: Pathogenesis, Protection, and Control. Washington, DC: ASM Press,
Tomioka H. Attempts to elucidate reasons why mycobacterial infections are intractable, by using an experimental mouse infection model.
Gomes MS, Appelberg R. Evidence for a link between iron metabolism and Nramp1 gene function in innate resistance against Mycobacterium avium.
Bosco MC, Rapisarda A, Reffo G et al. Macrophage activating properties of the tryptophan catabolite picolinic acid.
Blasi E, Mazzolla R, Pitzurra L et al. Protective effect of picolinic acid on mice intracerebrally infected with lethal doses of Candida albicans.
Akaki T, Sato K, Tomioka H et al. Effector molecules in expression of the antimicrobial activity of macrophages against Mycobacterium avium complex: roles of reactive nitrogen intermediates, reactive oxygen intermediates, and free fatty acids.
Akaki T, Tomioka H, Shimizu T et al. Comparative roles of free fatty acids with reactive nitrogen intermediates and reactive oxygen intermediates in expression of the anti-microbial activity of macrophages against Mycobacterium tuberculosis.
Hancock RE, Scott MG. The role of antimicrobial peptides in animal defenses.
Sharma S, Verma I, Khuller GK. Therapeutic potential of human neutrophil peptide 1 against experimental tuberculosis.
Beskid M, Jachimowicz J, Taraszewska A et al. Histological and ultrastructural changes in the rat brain following systemic administration of picolinic acid.
Alangaden GJ, Lerner SA. The clinical use of fluoroquinolones for the treatment of mycobacterial diseases.