-
PDF
- Split View
-
Views
-
Cite
Cite
E.R. Delay, N.P. Hernandez, K. Bromley, R.F. Margolskee, Sucrose and Monosodium Glutamate Taste Thresholds and Discrimination Ability of T1R3 Knockout Mice, Chemical Senses, Volume 31, Issue 4, May 2006, Pages 351–357, https://doi.org/10.1093/chemse/bjj039
Close - Share Icon Share
Abstract
Molecular and behavioral studies have identified heterodimers of the T1R family as receptors for detecting the tastes of sweet (T1R2 + T1R3) and umami (T1R1 + T1R3). However, behavioral studies have reported conflicting findings with T1R3 knockout (KO) mice. One study showed a complete or nearly complete loss of preference for sweet and umami substances by KO mice, whereas KO mice in another study showed only a partial reduction in preferences for sucrose and monosodium glutamate (MSG), the prototypical umami substance. The present experiments used psychophysical methods to assess how sensitive T1R1-KO mice are to sucrose and MSG and discrimination methods to determine if these mice could distinguish between the tastes of sucrose and MSG. Detection thresholds of T1R3-KO mice and wild-type (WT) C57Bl mice were nearly identical for sucrose and MSG. Mice of both genotypes were easily able to discriminate between the tastes of sucrose and MSG. When amiloride (a sodium channel blocker) was added to all solutions to reduce the taste of Na+, discrimination accuracy of both genotypes of mice decreased but more so for the T1R3-KO mice than the WT mice. However, even when the sodium taste of MSG was neutralized, both genotypes could still discriminate between the two substances well above chance performance. These results suggest that sucrose and MSG can be detected by taste receptors other than T1R2 + T1R3 and T1R1 + T1R3 and that the conflicts between the previous studies may have been due to the methodological limitations.
Introduction
Gustation or the sense of taste is important for locating food sources, maintaining nutritional equilibrium, and avoiding the ingestion of harmful substances. Although many chemical stimuli are capable of eliciting taste sensations, only a few of these sensations are considered primary tastes: sour, salty, bitter, sweet, and umami (Yamaguchi, 1967). Each primary taste is capable of influencing ingestive behavior by signaling a general food type. For example, sweet taste often signals carbohydrates such as sucrose, the prototypical sweet substance, and umami often signals the presence of dietary protein. Umami taste is elicited by monosodium glutamate (MSG), an amino acid that is a natural constituent of many protein-rich food items such as meats, cheeses, and vegetables. MSG has long been part of Asian cuisine and, in small quantities, is able to enhance flavor (Maga, 1983) and increase the palatability of food. These effects can have important health care implications when used to increase ingestion of nutritionally desirable foods in clinical populations (e.g., diabetic) who have dietary challenges (Bellisle, 1999).
The taste qualities of a chemical substance such as MSG are detected primarily by taste receptor cells in taste buds located in the tongue and soft palate of the mouth (Finger and Simon, 2000). The search for the membrane receptor or receptors capable of detecting sweet and umami tastes has been intense for several years. Recently, these efforts have identified potential candidates, primarily G protein–coupled receptors (GPCRs) of the T1R receptor family (Li et al., 2002; Nelson et al., 2002) and the mGluR family (Chaudhari et al., 1996, 2000), capable of detecting sweet and/or umami stimuli. For example, it is thought that when T1R receptors combine as specific heterodimers, the resulting receptors are selective for sweet and for umami tastes. Nelson et al. (2002) reported that the T1R1 + T1R3 heterodimer in vitro is activated by umami substances and most L-amino acids but is unresponsive to sweet stimuli. In contrast, T1R2 + T1R3 receptors respond to a wide variety of natural sugars and artificial sweeteners but not to umami substances. Zhao et al. (2003) developed a transgenic mouse in which the T1R3 receptor is knocked out and studied their preferences for taste substances with brief access tests. They found that these knockout (KO) mice showed no preference for umami substances or artificial sweeteners and had only minimal preference for high concentrations of natural sugars such as sucrose, suggesting that these mice had lost their ability to detect these substances. These findings are not without controversy, however. An independently developed T1R3-KO mouse also exhibited a complete loss of sensitivity for artificial sweeteners in two-bottle preference tests but only moderately reduced preference for natural sugars and MSG (Damak et al., 2003). Damak et al. also found that their T1R3-KO mice responded to natural sugars and MSG in nerve recording assays. The findings of the Damak et al. study open the possibility that there may be other receptors involved in the detection of sweet and umami stimuli.
Taste preference tasks can be excellent tools for studying the hedonic qualities of a taste stimulus, but these tasks may not accurately assess taste sensitivity if the subject does not prefer the taste qualities of the substance (Spector, 2003). For example, the T1R3-KO mice may be able to detect a substance but lack the ability to detect the qualities of the substance that make it more or less preferable relative to another substance, for example, water. In this case, more sensitive behavioral measures are required to assess the sensitivity of T1R3-KO mice to detect sucrose and MSG and to determine whether the KO mice are able to discriminate between qualitative features of these substances. If the T1R3 receptor is critical for sensing umami and sweet stimuli and for transducing qualitative features of these stimuli (Nelson et al., 2002; Damak et al., 2003; Zhao et al., 2003), then T1R3-KO mice should have substantially elevated detection thresholds for sucrose and MSG and should not be able to discriminate between the identifying taste qualities of sucrose and MSG.
Material and methods
Subjects
C57Bl/J6 wild-type (WT) mice and the T1R3 (−/−) KO mice, originally developed at Mount Sinai Medical School (Damak et al., 2003) from the C57Bl/6J strain, were reared at Colorado State University where the genetic deletion was verified by polymerase chain reaction (PCR). At the beginning of the experiment, all animals were 65–70 days old and were placed in separate cages for the duration of the experiment. Two weeks prior to testing, the mice were placed on a 22-h water deprivation schedule that was maintained throughout the experiment. Purina Lab chow was available ad libitum. The colony lighting was regulated according to a 12-h light/dark cycle with the lights turned on at 7:00 AM. All testing took place during the light portion of the cycle, and each mouse was tested at the same time each day.
Apparatus
The mice were tested with a computer-controlled Knosys Ltd gustometer (Brosvic and Slotnick, 1986). Each gustometer consisted of a Plexiglas operant chamber (17 × 12 × 12 cm) with a small circular opening in one wall, 1 cm in diameter, and centered 2 cm above the floor of the chamber. This opening gave the mouse access to a drinking spout positioned flush with the inner surface of the portal. Each taste solution was stored in one of eight 3-ml unpressurized syringe barrels at least 7.5 cm above the drinking spout. The flow of solution from each syringe barrel was regulated by solenoids, located at least 15 cm from the chamber. All syringe barrels were connected to capillary tubing through which each solution flowed to individual 24 gauge stainless steel tubes within the drinking spout. The tips of these tubes were recessed 2 mm from the end of the spout. Each taste stimulus was presented as a 6-μl aliquot delivered over 0.25 s. Licks were counted when the animal's tongue made contact with the drinking spout allowing a 65-μA current to flow through a stainless steel plate on the floor of the chamber. All testing was conducted in 30 ± 5 lx white fluorescent room light. To reduce auditory cues, an independent solenoid was activated simultaneously with the solenoid delivering the taste stimulus. In addition, a room fan, along with a Radio Shack Sleep Machine, generated masking noise (SPL A scale: 80 ± 5 dB) throughout the test period. A fan, mounted in the ceiling of the chamber, reduced olfactory cues by forcing air out of the chamber through the opening for the lick spout.
Procedures
General methods
Threshold and discrimination procedures were similar to those used previously (Stapleton et al., 2002; Delay et al., 2004). In these experiments, initiation of a trial occurred when the mouse licked on a variable ratio 20 schedule that resulted in a 3-μl water aliquot. This served to rinse the tongue and to encourage further licking. Three seconds later, the mouse began a second variable ratio 20 schedule which, when completed, resulted in the delivery of the 6 μl stimulus aliquot. Once the stimulus was delivered, the mouse had 2 s (decision interval) to determine if the stimulus was an S+ or an S−. After the delivery of an S+ solution, the mouse had to lick the spout during the last 0.4 s of the decision interval to receive a 7-μl water reinforcer (i.e., correct detection of the S+). Upon delivery of an S− solution, a correct detection was registered if the mouse did not lick during the last 0.4 s of the decision interval. If the animal failed to correctly respond to the S− taste stimulus, a weak shock was delivered through the lick spout to the animal's tongue. The shock intensity was adjusted for each animal by increasing the intensity until the mouse's licking stopped briefly when the shock was applied. Shock was always presented to the lick spout for 2 s following the end of the decision interval of S− trials, but the animal only experienced shock if it licked the spout during the shock presentation. A 10-s intertrial interval occurred before the start of the next trial. To minimize the possibility that a mouse could identify a taste stimulus using the location of the stimulus delivery in the spout, each day stimulus solutions were stored in different syringe barrels and a different concentration sequence, established from latin square procedures, was tested. The pH of all stimuli was between 6.75 and 7.0, and each solution was mixed fresh each day. A session ended after the animal completed 160 trials or 35 min elapsed, whichever came first. After the session ended, the mouse was returned to its home cage where 30 min later it received access to water for an additional 60 min.
Threshold procedures
To measure detection thresholds, six WT and five KO mice were randomly selected to learn to discriminate MSG (S− solution) from deionized water (S+ solution). Another six WT and five KO mice were assigned to the sucrose threshold experiment. During each training session, five of the stimulus barrels contained different concentrations of the taste stimulus (S−) and three contained deionized water (S+). An equal number of S+ and S− trials were presented within each session. Threshold training with the assigned S− began with concentrations of MSG or sucrose ranging from 200 to 500 mM. When the mouse was able to detect the highest concentration at >75% in three consecutive sessions, the range of concentrations was decreased in the next session by replacing the highest concentration with a new low concentration. Once the highest concentration was decreased to 150 mM, daily testing continued with 100 and 150 mM and with stimuli selected from two additional arrays. One stimulus was selected from 0.01, 0.1, 1.0, 2.5, 5, 10, and 15 mM, and two stimuli were selected from 25, 50, and 75 mM. Data collection began after performance stabilized and continued for a minimum of 20 days. To determine if Na+ interfered with the threshold for either MSG or sucrose, these procedures were then repeated with 100 μM amiloride added to all solutions, including water reinforcer. Amiloride is a sodium channel blocker (Heck et al., 1984) that is reported to raise sodium detection thresholds in WT C57Bl mice to over 500 mM (Eylam and Spector, 2003) and is tasteless to mice at 100 μM (Eylam et al., 2003). All concentrations were tested in at least two sessions under each amiloride condition.
Discrimination experiments
After thresholds for sucrose and MSG were estimated, six of the WT and all 10 of the KO mice began discrimination training. Two of the KO mice were eliminated from the experiment, one as a result of illness and the second because of an apparent loss of stimulus control. Discrimination procedures were initiated by changing the S+ condition from water to the opposite taste substance. For example, mice in the MSG threshold study were trained with sucrose as the S+ and MSG as the S−. Those mice in the sucrose threshold study were trained with MSG as the S+ and sucrose as the S−. To minimize the possibility that stimulus intensity rather than quality could serve as a discriminative cue and to ensure that the concentrations were well above the thresholds for each mouse, all testing was conducted with 100, 150, 200, and 300 mM of MSG and sucrose.
Because the Na+ ion of MSG could serve as a cue to differentiate MSG from sucrose, these experiments were conducted under three separate conditions to control for sodium taste: 1) no amiloride, 2) amiloride (100 μM) in all solutions, and 3) amiloride (100 μM) in all solutions and NaCl added to the sucrose solutions. Although amiloride has been reported to increase the threshold for NaCl to over 500 mM in C57Bl WT mice (Eylam and Spector, 2003), Ruiz et al. (2005) recently reported that amiloride may not be fully effective at elevating sodium thresholds under these experimental procedures. Therefore, to ensure that the cue function of sodium taste of MSG was neutralized, NaCl was added to each solution of sucrose to match the Na+ content of each concentration of MSG (Stapleton et al., 2002; Heyer et al., 2004). Thus, 10 mM of NaCl was added to 10 mM sucrose to match the sodium concentration of 10 mM MSG, and so on.
To ensure stable performance, discrimination training began with at least 20 days with the no amiloride condition. The mice were then run an additional 4 days for data collection. Half of the mice in each group were then given 10 days of training with the amiloride condition and then 4 days of testing. This was followed by another 10 days of training and 4 days of testing with the amiloride and NaCl matching condition. The rest of the mice were tested with the opposite order of amiloride/amiloride plus NaCl conditions. Finally, all mice were retested in the no amiloride condition for 10 days. Data from the last 4 days were averaged with the scores for the first no amiloride condition to control for experience. After completion of each amiloride condition, one additional test session was conducted to determine if any of the mice were able to discriminate between stimulus tubes on the basis of nongustatory cues such as spout location or some equipment generated cue. All experimental parameters were maintained during this session except each tube was filled with water and randomly assigned as an S+ or S−.
Results
Threshold experiments
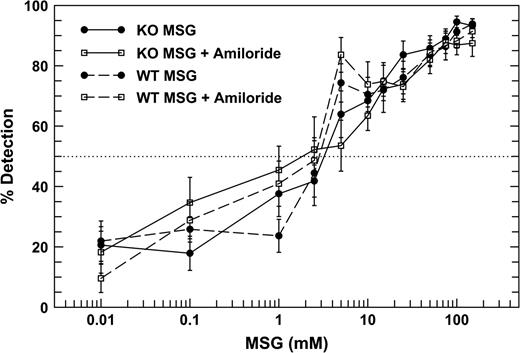
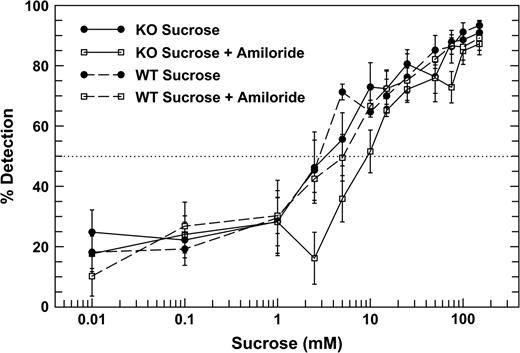
The detection thresholds for MSG and sucrose, defined as the concentration detected 50% of the time, were determined for each mouse by interpolation using log scaling. These data were then subjected to an analysis of variance for mixed designs to compare thresholds of the genotypes in the two amiloride conditions. Figures 1 and 2 show the psychophysical functions for each genotype mouse for MSG and sucrose without amiloride and mixed with 100 μM amiloride. MSG thresholds for the WT mice were 2.40 mM ± 0.54 (geometric mean ± SEM) without amiloride and 1.23 mM ± 0.36 with amiloride added. MSG thresholds of the T1R3-KO mice were 3.01 ± 1.45 mM without amiloride and 2.01 ± 1.93 mM with amiloride (Figure 1). Sucrose thresholds for the WT mice were 1.93 mM ± 0.51 mM without amiloride and 3.93 ± 1.08 mM with amiloride added (Figure 2). For the KO mice, sucrose thresholds were 3.46 ± 1.91 mM without amiloride and 7.89 ± 1.48 mM with amiloride. Importantly, no significant differences in thresholds were found between mice of the two genotypes, either in the presence or absence of amiloride.
Psychophysical functions of the WT (dashed lines) and KO (solid lines) mice for MSG without and with 100 μM amiloride in all solutions. No significant differences between the thresholds of KO and the WT mice were detected.
Psychophysical functions of the WT (dashed lines) and KO (solid lines) mice for sucrose without and with 100 μM amiloride in all solutions. No significant differences between the thresholds of KO and the WT mice were detected.
Discrimination experiments
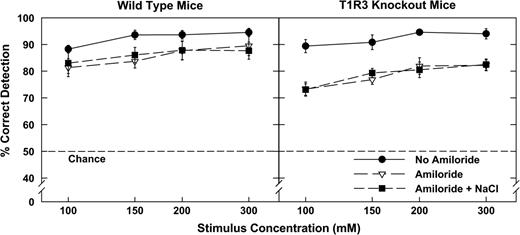
The analysis of variance (ANOVA) of the data for WT mice indicated that, as expected, these mice were able to discriminate sucrose from MSG significantly more accurately as the test concentrations increased [F(3,21) = 17.16, P < 0.001]. The taste of Na+ contributed insofar as the WT mice detected significantly more taste stimuli in the no amiloride condition than in either the amiloride or amiloride plus NaCl conditions (Figure 3, left panel) [F(2,10) = 4.355, P < 0.01]. Similar findings were identified in the data for the T1R3-KO mice. That is, performance was significantly better at higher concentrations than lower concentrations. Detection rates were also significantly better in the no amiloride condition than in the other two conditions in which amiloride was added (Figure 3, right panel) [F(2,14) = 40.20, P < 0.001].
Percent correct detections for matched concentrations of sucrose and MSG in three Na+ cue conditions: 1) no amiloride, 2) amiloride (100 μM), and 3) amiloride (100 μM) plus NaCl added to sucrose to match the concentration of MSG. Mice could discriminate between MSG and sucrose, but they had more difficulty when amiloride was added. WT mice (left panel) were better than the KO mice (right panel) at discriminating between sucrose and MSG tastes when amiloride was added.
The data were then partitioned to determine if there were significant differences between genotypes under the three amiloride conditions to control for sodium taste. ANOVA procedures did not find any significant differences between the KO and WT mice in the no amiloride condition. Since the scores for the amiloride and amiloride plus NaCl conditions were virtually identical for both groups, they were analyzed together with a two-factor ANOVA for mixed designs. This analysis indicated that the WT mice discriminated between the two taste substances significantly more accurately than the KO mice when the cue function of sodium is reduced or neutralized [F(1,12) = 12.00, P < 0.001]. Additional t-tests verified the better discrimination rates of WT in the amiloride (P < 0.039) and in the amiloride plus NaCl conditions (P < 0.034).
Discussion
The data reported in this study show that T1R3-KO mice are able to detect MSG and sucrose at concentrations comparable to those detected by WT mice. More surprisingly, KO mice were also able to discriminate between the taste qualities of MSG and sucrose, even when the cue function of the sodium component of MSG was reduced or neutralized. T1R3-KO mice were only somewhat less capable than WT mice of discriminating MSG from sucrose when amiloride was present. These results, in conjunction with previous reports on the behavioral and nerve responses of T1R3-KO mice (Damak et al., 2003), suggest that the taste capacities of T1R3-KO mice for sucrose and MSG are altered but only moderately diminished relative to their WT counterparts and that additional taste receptors are involved.
Earlier conditioned taste aversion studies (Kasahara et al., 1987; Yamamoto et al., 1991; Heyer et al., 2003) suggested that sucrose and MSG share common taste qualities and that the taste of MSG may simply be equivalent to the taste of “sweet” (by human standards). However, more recent behavioral research (Stapleton et al., 2002; Heyer et al., 2004) showing that rats easily discriminate between MSG and most sweet substances argued against this. Discrimination methods such as those used in this study force the animal to attend to differences in stimulus qualities rather than similarities in taste qualities. In this study, WT C57Bl mice easily discriminated between the tastes of sucrose and MSG. When the taste of sodium was reduced or neutralized, forcing the WT mice to focus on the tastes of sucrose and the glutamate anion of MSG, performance declined significantly. Even so, these animals were still capable of distinguishing between these substances at rates above chance, suggesting that glutamate and sucrose elicit similar but not identical taste sensations in WT mice. These results, in conjunction with behavioral studies of rats (see e.g., Delay et al., 2000, 2004) and behavioral and nerve recording studies of WT and KO mice (see e.g., Damak et al., 2003; Zhao et al., 2003), argue that the perceptual experience elicited by glutamate in C57Bl WT mice is not simply sweet.
Minimizing the cue function of sodium hampered the discrimination ability of KO mice more than that of WT mice. Even so, the T1R3-KO mice were capable of discriminating between sucrose and MSG well above chance levels. Molecular studies with heterologously expressed T1R receptors (Li et al., 2002; Nelson et al., 2002) and limited behavioral studies with T1R3-KO mice (Zhao et al., 2003) indicated that the T1R3 receptor is essential for functional sweet and umami taste receptors. The prediction from these studies is that without T1R3, T1R3-KO mice should not be able to distinguish between the taste qualities of prototypical sweet substances such as sucrose and umami substances such as MSG. That is, even if T1R3-KO mice are able to detect these substances, they lack the ability to encode information about the qualitative features of sweet and umami taste substances. The data reported in the present study, however, do not support this position. Despite the deletion of the Tas1r3-encoded T1R3 subunit, during a trial the T1R3-KO mice were able, albeit less effectively than WT mice, to ascertain enough unique qualitative taste information about each substance to associate it with the learned hedonic consequence. These results suggest that the T1R3-KO mice used T1R3-independent mechanisms to differentiate between the two substances.
This conclusion is also supported by the threshold findings. Thresholds for sucrose and MSG measured for both mouse genotypes in this study are comparable to thresholds measured for Sprague-Dawley rats (Campbell, 1958; Sclafani and Nissenbaum, 1987a; Thaw, 1996; Bachmanov et al., 2001; Stapleton et al., 2002). If the T1R3 receptor is critical for detecting sweet and umami substances, then one might expect that deletion of the Tas1r3 gene should raise detection thresholds for sucrose and MSG, perhaps by several log units. However, the taste thresholds of mice for these substances appear to be unaffected by the genetic ablation of T1R3. These findings clearly contradict the proposal that heterodimeric combinations of T1R3 with other T1R receptors are necessary and sufficient for detection of sweet and umami substances in vitro (Li et al., 2002; Nelson et al., 2002) and in vivo (Zhao et al., 2003) but are consistent with other research findings indicative of T1R3-independent detection of sweet and umami compounds (e.g., Chaudhari et al., 1996, 2000; Damak et al., 2003; Nie et al., 2005).
How can we reconcile the results of Zhao et al. (2003), who observed a complete or nearly complete loss of preference for sucrose and MSG in T1R3-KO mice, and those of Damak et al. (2003), who reported reduced preferences for sucrose and MSG in their T1R3-KO mice? Further, how can we reconcile those results with the current experiments showing no loss of sensitivity and only a modest loss of discriminability of MSG and sucrose in the T1R3-KO mice of Damak et al. (2003)? One possibility may be due to differences in the T1R3-KO constructs used by each of the groups. Damak et al. (2003) removed the entire coding region of T1R3, whereas Zhao et al. (2003) removed only that portion of the N-terminal extracellular domain contained within exons 1–5 but retained the remainder of the gene (i.e., part of cysteine-rich region and the entire heptahelical transmembrane domain). Both types of gene targeting events would likely render the Tas1r3 gene nonfunctional. However, if a truncated form of T1R3 containing the transmembrane domain were to be expressed, then it might be capable of dimerizing with T1R2 and T1R1 and inhibiting their responses to sucrose and MSG, respectively, that is, it would act as a dominant negative. Another possibility might be due to differences in the behavioral methods used by these two groups. Zhao et al. (2003) used a brief access test (Glendinning et al., 2002) that appears to be able to assess the hedonic quality of, or preference for, a taste stimulus with minimum postingestive effects (Spector, 2003). If the deletion of the Tas1r3 gene eliminated a component of transduction that is important for signaling the hedonic quality for the mouse, the KO mouse may be able to readily detect the substance but, without sufficient hedonic signal, lacks the motivation to alter its behavior when the mouse encounters sucrose or MSG. Damak et al. (2003) used 24-h two-bottle preference tests to assess the taste capacities of their T1R3-KO mouse. While this test is able to identify preferences for a substance, it is well known that the behavioral outcome can be significantly altered by postingestive effects of a substance (Sclafani and Nissenbaum, 1987b; Ackroff and Sclafani, 1994; Spector, 2003). For example, sucrose may have induced a positive physiological state that the T1R3-KO mouse associated with the altered signal of sucrose that resulted in an increase in consumption of sucrose relative to water. In the present study, the pairing of reinforcement with one substance and aversion with the second substance encourages the mouse to assign a hedonic value to each substance, which in this paradigm, is independent of natural hedonic qualities or postingestive effects. However, to be able to do this, the T1R3-KO mouse must first be able to detect unique taste qualities for each substance before it can form the necessary association with the assigned response consequence. Thus, the discrimination methods employed in this study, in contrast to the preference methods used by Damak et al. (2003) and Zhao et al. (2003), force the T1R3-KO mice to use T1R3-independent receptors that may be less efficient or less important than the T1R3-dependent receptors functioning in WT mice.
What can we infer of the nature of the T1R3-independent mechanisms for detection and discrimination of sweet and umami stimuli? These mechanisms could include receptors that normally function in combination with T1R3 but which in the absence of T1R3 might function on their own (as monomers or as homodimers) or in combination with other receptors (as heterodimers). Thus, although T1R2 + T1R3 and T1R1 + T1R3 might normally function to detect sucrose and MSG, respectively, in the absence of T1R3, homodimers of T1R2 or of T1R1 might respond to sucrose and MSG, respectively. Support for this idea comes from the recent report that when individually expressed, the extracellular portions of T1R2 or T1R3 bind sucrose with nearly the same affinity as does the heterodimeric T1R2 + T1R3 sweet receptor (Nie et al., 2005). That is, T1R2 might function on its own as a sugar detector. Another possibility for T1R3-independent detection mechanisms could be that other non-T1R receptors expressed in taste cells respond to sucrose and/or MSG. Taste expressed mGluR4 and mGluR1 might constitute such receptors. Chaudhari et al. (2000) cloned a family C metabotropic glutamate receptor, taste-mGluR4, which has response characteristics that correspond well with behavioral features of glutamate taste (Chaudhari et al., 1996; Delay et al., 2000, 2004). Others have reported evidence that mGluR1 and mGluR5 receptors may also be involved in taste (Toyono et al., 2003). It is also possible that in the absence of T1R3, atypical heterodimers of family C GPCRs form (e.g., T1R2 + mGluR1 or T1R1 + mGluR4). These atypical heterodimers might provide for detection and even discrimination of MSG and sucrose, but perhaps not with the normal fidelity displayed by WT mice. Calcium imaging studies performed on taste cells from WT and T1R3-KO mice stimulated with umami stimuli support the idea of taste signaling mechanisms occurring in the absence of T1R3 (Maruyama et al., 2006).
In summary, T1R3-KO mice have detection thresholds for MSG and sucrose comparable to their WT counterparts. T1R3-KO mice were also able to discriminate between the tastes of glutamate and sucrose, although not as well as the WT mice; this discrimination occurred even when the cue function of sodium taste of MSG was controlled. These findings, in conjunction with other studies, indicate that the T1R3-KO mice are able to utilize T1R3-independent means to identify and discriminate between glutamate and sucrose.
The authors wish to thank Dr Sue Kinnamon for maintaining the T1R3-KO mouse colony at Colorado State University. The authors also wish to thank Collin Ruiz for performing the PCR screening, Beth Heyer for conducting preliminary studies with the T1R3-KO mice, Lee Hernandez-Ball for his participation in these experiments, and Dr Stephen Roper for a critical review of the manuscript. These experiments were funded by National Institutes of Health grants DC5962 and DC7617 awarded to E.R.D. and DC3155 awarded to R.F.M.
References
Ackroff, K. and Sclafani, A. (
Bachmanov, A.A., Tordoff, M.G. and Beauchamp, G.K. (
Bellisle, F. (
Brosvic, G.M. and Slotnick, B.M. (
Campbell, B.A. (
Chaudhari, N., Landin, A.M. and Roper, S.D. (
Chaudhari, N., Yang, H., Lamp, C., Delay, E., Cartford, C., Than, T. and Roper, S. (
Damak, S., Rong, M., Yasumatsu, K., Kokrashvili, Z., Varadarajan, V., Zou, S., Jiang, P., Ninomiya, Y. and Margolskee, R.F. (
Delay, E.R., Beaver, A.J., Wagner, K.A., Stapleton, J.R., Harbaugh, J.O., Catron, K.D. and Roper, S.D. (
Delay, E.R., Sewczak, G.M., Stapleton, J.R. and Roper, S.D. (
Eylam, S. and Spector, A.C. (
Eylam, S., Tracy, T., Garcea, M. and Spector, A.C. (
Finger, T.E. and Simon, S.A. (
Glendinning, J.I., Gresack, J. and Spector, A.C. (
Heck, G.L., Mierson, S. and DeSimone, J.A. (
Heyer, B.R., Taylor-Burds, C.C., Mitzelfelt, J.D. and Delay, E.R. (
Heyer, B.R., Taylor-Burds, C.C., Tran, L.H. and Delay, E.R. (
Kasahara, T., Iwasaki, K. and Sato, M. (
Li, X., Staszewski, L., Xu, H., Durick, K., Zoller, M. and Adler, E. (
Maruyama, Y., Pereira, E., Margolskee, R.F., Chaudhari, N. and Roper, S.D. (
Nelson, G., Chandrashekar, J., Hoon, M.A., Feng, L., Zhao, G., Ryba, N.J.P. and Zuker, C.S. (
Nie, Y., Vigues, S., Hobbs, J.R., Conn, G.L. and Munger, S.D. (
Ruiz, C., Gutknecht, S., Mahaffey, J., Delay, E. and Kinnamon, S. (
Sclafani, A. and Nissenbaum, J.W. (
Sclafani, A. and Nissenbaum, J.W. (
Spector, A.C. (
Stapleton, J.R., Luellig, M., Roper, S.D. and Delay, E.R. (
Thaw, A.K. (
Toyono, T., Seta, Y., Kataoka, S., Kawano, S., Shigemoto, R. and Toyoshima, K. (
Yamaguchi, S. (
Yamamoto, T., Matsuo, R., Fujimoto, Y., Fukunaga, I., Miyasaka, A. and Imoto, T. (
Author notes
1Neuroscience Program, Department of Psychology, Regis University, Denver, CO, USA, 2Department of Biology, University of Vermont, Burlington, VT, USA and 3Department of Neuroscience, Mount Sinai School of Medicine, New York, NY, USA