Abstract
3,4-Methylenedioxy-N-methamphetamine (MDMA or ‘ecstasy’) is a psychoactive substance, first described as an appetite suppressant in humans, inducing side effects and even death. MDMA increases serotonin (5-HT) levels, and 5-HT inhibits food intake, but the 5-HT receptors involved in MDMA-induced changes in feeding behavior are unknown. We examined whether a systemic MDMA injection would reduce the physiological drive to eat in starved mice and tested if the inactivation of 5-HT1B or 5-HT2C receptors could restore this response. Our results indicate that in starved mice, MDMA (10 mg/kg) provoked an initial hypophagia for 1 h (−77%) followed by a period of hyperphagia (studied between 1 and 3 h). This biphasic feeding behavior due to MDMA treatment was maintained in 5-HT1B receptor-null mice or in animals treated with the 5-HT1B/1D receptor antagonist GR127935 (3 or 10 mg/kg). In contrast, MDMA-induced hypophagia (for the first 1 h period) was suppressed when combined with the 5-HT2C receptor antagonist RS102221 (2 mg/kg). However, RS102221 did not alter MDMA-induced hyperphagia (for the 1–3 h period) but did exert a stimulant effect, when administered alone, during that period. We have previously shown that MDMA or 5-HT1A/1B receptor agonist RU24969 fails to stimulate locomotor activity in 5-HT1B receptor-null mice. Our present data indicate that the 5-HT2C receptor antagonist RS102221 suppresses MDMA-induced hyperlocomotion. These findings provide the first evidence that the inactivation of 5-HT2C receptors may reduce hypophagia and motor response to MDMA, while a genetic deficit or pharmacological inactivation of 5-HT1B receptors was insufficient to alter the feeding response to MDMA.
Similar content being viewed by others
INTRODUCTION
It is well known that serotonin (5-HT) regulates feeding behavior, as shown by the potent anorectic properties of 5-HT releasers, such as fenfluramine. Pharmacological studies combined with knockout strategies have clearly demonstrated that among the 15 5-HT receptor subtypes, the 5-HT1B and 5-HT2C receptors are key elements regulating food intake in mammals. The 5-HT1B, 5-HT2A, and 5-HT2C receptor agonists produce hypophagia (Bendotti and Samanin, 1987; Kennett and Curzon, 1988; Schechter and Simansky, 1988; Macor et al, 1990; Aulakh et al, 1994; Halford and Blundell, 1996; Lee and Simansky, 1997; Lucas et al, 1998; Vickers et al, 2001; Lee et al, 2004). Conversely, the inactivation of 5-HT2C receptors alters fenfluramine-induced anorexia (Neill and Cooper, 1989; Vickers et al, 1999, 2001; Heisler et al, 2002). 5-HT2C receptor-null mice, known for being obese (Tecott et al, 1995), display reduced sensitivity to fenfluramine (Vickers et al, 1999, 2001). The possible roles of 5-HT1B receptors in d-fenfluramine-induced anorexia are more complex. Acute treatment with GR127935, a 5-HT1B receptor antagonist, or with cyanopindolol, a 5-HT1A/1B receptor antagonist, does not modify d-fenfluramine-induced anorexia in starved rats (Vickers et al, 2001). In contrast, 5-HT1B receptor-null mice are less sensitive to d-fenfluramine than wild-type mice (Lucas et al, 1998).
Among drugs that increase synaptic levels of 5-HT, antidepressants are known clearly to modify food intake, whereas the action of some others such as 3,4-N-methylenedioxymethamphetamine (MDMA or ‘ecstasy’) have not been investigated. This is surprising since MDMA is a widely used recreational drug. MDMA is defined as a ‘substrate-type 5-HT releaser’ (Rothman and Baumann, 2002) and is an amphetamine-like stimulant. MDMA (10 mg/kg) increases the levels of 5-HT, but can also increase dopamine (DA) levels, especially at higher doses (Colado et al, 2004).
The primary goals of this study were to investigate whether MDMA (10 mg/kg) could counteract starvation-induced eating and whether the 5-HT1B and 5-HT2C receptors were involved in this effect.
MATERIALS AND METHODS
Animals
All experiments were performed on male adult wild-type and 5-HT1B receptor-null mice on a 129/Sv genetic background (see Phillips et al, 1999 for a description of the exact genetic background of the mice), aged 4–6 months. The 5-HT1B receptor-null mice were generated as described previously (Saudou et al, 1994). Both the wild-type and null mice were all obtained from heterozygous breeding at the transgenic animal facility of the UPR CNRS 5203 in Montpellier (France). The genotype of each mouse was identified using the PCR technique. For the 5-HT2C receptor experimental paradigms, wild-type mice on a 129/Sv (Iffa Credo, France) were used at 4–6 months old. Mice were housed five per cage with food and water available ad libitum and maintained in a temperature-controlled environment on a 12-h light/dark cycle with light onset at 06:00. The experiments were carried out in accordance with the Guide for Care and Use of Laboratory Animals established by the National Institutes of Health of the United States of America.
Procedures
In summary, three different sets of experiments were performed using the feeding paradigm described in detail below and previously used by Lucas and co-workers (1998). First, the wild-type and 5-HT1B receptor-null mice were treated with a single intraperitoneal (i.p.) injection of saline, MDMA, or 5-HT1B receptor agonist. Second, another group of wild-type mice was similarly injected with NaCl, MDMA, or 5-HT1B receptor antagonist GR127935 either alone or in combination with MDMA. Third, NaCl, MDMA, or 5-HT2C receptor antagonist RS102221 alone or combined with MDMA was administered in the same manner to a naïve group of wild-type mice. Finally, four additional groups of wild-type mice were treated with NaCl, MDMA, or RS102221 either alone or coadministered with MDMA and tested in open field chambers (Activity section), as described previously (Compan et al, 2003, 2004). In each experimental paradigm, animals were treated with an equivalent volume (3.3 ml/kg) of vehicle via the same route.
Feeding Paradigm Tests
The feeding procedure followed was exactly the same as the one used by Lucas and co-workers for fenfluramine (1998). At 3 days before the experiments, 5-HT1B receptor-null and wild-type mice were housed singly with ad libitum access to classic food (pellet form, 16.5% crude proteins, 3.6% crude fat, crude fiber 4.6%, ash 5.2%) and tap water. Animals were then food deprived for 24 h with water given ad libitum (09:00 to 21:00), as previously used in mice (Lucas et al, 1998) and rats (23 h, Vickers et al, 2001). Tap water consumption was not measured. On the testing day, classic food was reintroduced into the trough 10 min after an acute i.p. injection of one of the treatments described above. Food was briefly removed (<20 s) and weighed at 1 and 3 h following the initial reintroduction. First, we tested the effects of MDMA (10 mg/kg) in mice of both genotypes. To make sure that our results did not depend on different experimentalists or laboratory conditions, other groups of mice of both genotypes were also treated with the 5-HT1A/1B receptor agonist RU24969 (5 mg/kg), as used by Lucas and co-workers (1998). In parallel, we assessed for the first time the effects of the selective 5-HT1B receptors agonist CGS12066A (0.5, 1 or 2 mg/kg) in wild-type and null mice. Second, wild-type mice received a 3, 5, or 10 mg/kg dose of the 5-HT1B/1D receptor antagonist GR127935 coadministered with or without MDMA (10 mg/kg). Third, a series of experiments were conducted following the injection of a 1 or 2 mg/kg dose of the selective 5-HT2C receptor antagonist RS102221 that was combined or not to MDMA (10 mg/kg) in wild-type mice.
In each experiment, animals received an i.p. injection of sterile 0.9% NaCl. Food intake and body weight were measured daily using a Sartorius CP32S balance (1 mg precision), which automatically provides the mean body weight of 10 values for each animal in movement.
Pharmacological Treatments
MDMA HCl (10 mg/kg, Sigma), 5-HT1A/1B agonist 5-methoxy-3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indole (RU24969; generously supplied by Roussel-Uclaf, Romainville, France), 5-HT1B agonist 7-trifluoromethyl-4(4-methyl-1-piperazinyl)-pyrrolo[1,2-a]quinoxaline maleate (CGS12066A; 0.5, 1 and 2 mg/kg, Sigma), 5-HT1B/1D receptor antagonist 2′-methyl-4′-(5-methyl-[1,2,4]oxadiazol-3-yl)-biphenyl-4-carboxylic acid [4-methoxy-3-(4-methyl-piperazine-1-yl)-phenyl]amide (GR127935; 3 and 10 mg/kg, generously provided by Glaxo Welcome, Taplow, UK), the selective 5-HT2C receptor antagonist 8-[5-(2,4-dimethoxy-5-(4-trifluoromethylphenylsulfonamido)phenyl-5-oxopentyl)]-1,3,8-triazaspiro[4.5]decane-2,4-dione hydrochloride (RS102221, 1 and 2 mg/kg, Tocris) were freshly dissolved in sterile 0.9% NaCl before their i.p. injection, on the day of testing.
Activity
Open-field test
Naïve male mice were tested for 3 h following NaCl, MDMA (10 mg/kg), RS102221 (2 mg/kg dose), or MDMA combined with RS102221. Testing was conducted between 09:00 and 17:00. The open-field test environment is a square chamber with an inside area that measures: 43.2 × 43.2 × 30.5 cm3. Mice were placed in the center and monitored with 32 infrared light sources spaced 0.5 in apart (1.25 cm) (MED, Associates, Inc., USA) specifically adapted to record the location, the traveled path length, and vertical counts. Two trained experimentalists, who were unaware of which drug was used, scored the number of behavioral stereotypy exhibited for each mouse, rated using the rating scale described by Marona-Lewicka and Nichols (1994) with some modification. Briefly, flat body posture including stretch–attend posture (the mouse stretches its body forward and backward without locomotion), slow stereotyped head weaving (the number of times the mice made a slow, side to side or lateral movement), licking, and grooming were determined using the following scoring scale: 1=1–4 times, 2=5–10 times, 3=11–20 times, and 4=more than 20 times. In addition, grooming and licking were counted for each animal. Such a study was not performed for 5-HT1B receptor-related experimental paradigms because we have previously performed detailed analyses indicating that MDMA (10 mg/kg)-induced locomotion is reduced in 5-HT1B receptor-null mice (Scearce-Levie et al, 1999; Compan et al, 2003).
Data Analysis
Data were analyzed using Statview 5 (Abacus concept, Berkeley, USA). For each set of experiments (Procedure section), a repeated measures ANOVA was systematically performed on the data, which were obtained in multiple sessions over time. Genotype and treatment were used as independent variables. Food intake or activity parameters were used as dependent variables. If significant effects of genotype or treatment, or a genotype and treatment interaction, over time or not, were found, the independent variables were split for a two- (genotype and treatment) or one-way ANOVA (genotype or treatment) analysis. Following a significant one-way ANOVA, we used the Scheffé F-test for multiple comparisons, with a probability of 0.01 and 0.05 defined as a significant difference. In order to simplify the presentation in the Results section, only the results from the separate ANOVAs conducted on each time period are described.
RESULTS
Similar Baseline Food Intake and Body Weight of 5-HT1B Receptor-Null and Wild-Type Mice Born from Couples of Heterozygous Animals
Two earlier studies have reported that male 5-HT1B receptor-null mice are overweight and consume a higher amount of food than wild-type mice (Brunner et al, 1999; Bouwknecht et al, 2001). However, the validity of the results was a matter of debate because wild-type and null mice were obtained from independent lines (Bouwknecht et al, 2001). Therefore, we used homozygote offspring from heterozygote breeding pairs (Animal section) in order to avoid indirect effects of parental care behavioral responses, as discussed by Bouwknecht et al (2001). No difference in either baseline food intake or body weight was detected between wild-type and 5-HT1B receptor-null mice for the habituation period or following a 24 h period of food deprivation (data not shown), as reported previously (Lucas et al, 1998).
Kinetics of Feeding Responses after MDMA Treatment in Starved Wild-Type Mice was Biphasic (Hypophagia Followed by Hyperphagia), and Contrasted to a Monophasic Hypophagia Response to 5-HT1B Receptor Agonists
MDMA produced a profound reduction in food intake for 1 h in starved wild-type mice, as compared with NaCl-treated wild-type mice (−77%, Figure 1a). However, after this initial reduction in food intake, we observed that the amount of food consumed over the next 2 h (between 60 and 180 min) was greater in MDMA-treated mice than in NaCl-injected wild-type mice (+47%, Figure 1b). Therefore, the drive to eat induced by starvation was suppressed during the first hour by MDMA. Compared to saline-treated starved mice, MDMA-treated starved mice first displayed a period of hypophagia for 1 h and then a second period of hyperphagia for the next 2 h.
Biphasic feeding responses to MDMA in starved wild-type and 5-HT1B receptor-null mice contrasted to a sustained hypophagia of 5-HT1B receptor agonists. Data are means±SEM total food intake for groups of wild-type (+/+) and 5-HT1B receptor-null (−/−) mice treated with NaCl (n=22–17), MDMA (10 mg/kg, n=11–9), 5-HT1A/1B receptor agonist RU24969 (5 mg/kg, n=9–7), or 5-HT1B receptor agonist CGS12066A (2 mg/kg, n=11–7). Food intake was significantly different between mice of both genotypes (F1,85=6.2, p<0.05) and changed after the treatment used for 1 h (F3,85=23.4, p<0.0001) and 60–180 min periods (F3,85=13.5, p<0.0001). Treatments that differ significantly from saline in wild-type mice are marked (§§§p<0.0001, §§p<0.01, §p<0.05) and, in 5-HT1B receptor-null animals, are noted (**p<0.01).
As with MDMA, the 5-HT1B receptor agonist CGS12066A (2 mg/kg, but not 0.5 or 1 mg/kg) reduced food consumption during the first hour following treatment (−65%, Figure 1a). However, in contrast to MDMA, CGS12066A-induced hypophagia was prolonged over 3 h (Figure 1b). Similar results were obtained using the 5-HT1A/1B receptor agonist RU24969 (5 mg/kg, Figure 1a and b), as reported previously (Lucas et al, 1998). Thus, 5-HT1B receptor agonists produced a more sustained hypophagia than does MDMA (Figure 1b).
Biphasic Feeding Responses to MDMA were Maintained in 5-HT1B Receptor-Null Mice
Since both MDMA and 5-HT1B receptor agonists reduced food intake in starved mice, we thought that 5-HT1B receptors could be involved in MDMA-delayed eating. However, we did not verify this hypothesis since, in 5-HT1B receptor-null mice, MDMA induced a biphasic effect on feeding behavior similar to the one observed in wild-type mice: a hypophagic effect over 1 h (−58%, Figure 1a) followed by a hyperphagic effect (Figure 1b). As expected, note that, no significant effect of CGS12066A (2 mg/kg) or RU24969 (5 mg/kg) injected alone was observed in 5-HT1B receptor-null mice (Figure 1a and b).
Biphasic Feeding Responses to MDMA were Maintained when 5-HT1B Receptors were Blocked by the Antagonist GR127935
To circumvent any compensatory mechanisms, which may occur in mice lacking 5-HT1B receptors from birth, we tested whether an acute injection of the specific 5-HT1B/1D receptor antagonist, GR127935 (10 mg/kg), combined with MDMA may disrupt the effects of MDMA on feeding. Results indicate that in starved wild-type mice, MDMA plus GR127935 did not modify the biphasic change in food intake provoked by MDMA (Figure 2a and b). In addition, no significant effect on food intake was detected in mice treated with 3 (not shown) or 10 mg/kg of GR127935, as compared to saline-injected wild-type animals (Figure 2a and b).
Biphasic feeding responses to MDMA was maintained even when the drug is coadministered with the 5-HT1B/1D receptor antagonist GR127935. Data are means±SEM total food intake measured 1 h (a) and between 1 and 3 h (b) in wild-type mice treated with NaCl (n=10), MDMA (10 mg/kg, n=9), 5-HT1B/1D receptor antagonist GR127935 (10 mg/kg, n=8), or MDMA/GR127935 (n=10) in starved wild-type mice. Significant differences between any treatment and NaCl effect are noted (§§§p<0.0001, §p<0.05).
5-HT2C Receptor Antagonist RS102221 Highly Reduced MDMA-Induced Hypophagia, but not Its Hyperphagic-Delayed Response
5-HT2C receptor-null mice exhibit an increase in food intake (Tecott et al, 1995), unrelated to peripheral change (Nonogaki et al, 1998). Pharmacological studies have reported that the 5-HT2C receptor antagonist SB242084 reduced the anorectic effects of both m-chlorophenylpiperazine, a mixed 5-HT2A,2B,2C receptor agonist (Kennett et al, 1997), and fenfluramine (Vickers et al, 2001). However, SB242084 alone did not induce hyperphagia, nor did it mimic other behavioral parameters of 5-HT2C receptor-null mice (Kennett et al, 1997). Therefore, we chose to use another selective 5-HT2C receptor antagonist, RS102221, because its chronic administration mimics the feeding phenotype of 5-HT2C-null mice (Bonhaus et al, 1997).
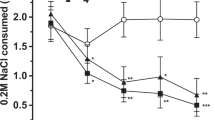
In this new series of experiments, the treatments induced significant changes in food intake over time (1 h: F3,36=12.5, p<0.0001; 60–180 min: F3,36=8.3, p<0.001). Again, we observed a biphasic change in food intake following MDMA injection in starved wild-type animals (Figure 3a and b). RS102221 (2 mg/kg) alone did not change the feeding response for the first period (0–60 min, Figure 3a). Interestingly, if RS102221 (2 mg/kg) was coadministered with MDMA, the hypophagic effect of MDMA observed during this period was greatly reduced compared to the one observed after injection of MDMA alone (Figure 3a). In contrast, for the second period (60–180 min), the hyperphagia detected after MDMA was not reduced after coinjection of RS102221 and MDMA (Figure 3b). Note that RS102221 injected alone induced hyperphagia during this period (Figure 3b).
5-HT2C receptor antagonist RS102221 coadministered with MDMA-suppressed MDMA-induced hypophagia, but not hyperphagia. Data are means±SEM total food intake in starved wild-type mice treated with NaCl (n=9), MDMA (10 mg/kg, n=10), 5-HT2C receptor antagonist RS102221 (2 mg/kg, n=12), or MDMA/RS102221 (2 mg/kg, n=13). Treatments that differ significantly from saline are marked (§§p<0.01, §p<0.05), and those differing from MDMA are noted (##p<0.01).
MDMA did not Induce Hyperlocomotion when Coinjected with RS102221
There is an obvious correlation between horizontal locomotion and feeding responses. To our knowledge, the possible involvement of 5-HT2C receptors in MDMA-induced hyperactivity has never been tested in mice. Our results indicate that MDMA significantly increased the traveled path length, as compared to saline-injected animals (Figure 4a). In contrast, a combined treatment of MDMA plus RS102221 failed to induce any change in horizontal activity (Figure 4a). Similarly, the total traveled path length was markedly increased after the administration of MDMA, as compared to control mice (+592%, Figure 4b). This MDMA-induced activity was highly reduced by the coinjection of RS102221 (+226%, p<0.01) (Figure 4a and b). Note that RS102221 alone had no effect on locomotor activity (Figure 4a and b).
A combined MDMA treatment with the 5-HT2C receptor antagonist RS102221 induced no change in the open field. Näive wild-type animals were placed in identical open fields and their horizontal activity (a–c) and stereotyped behaviors (flat body and stretch–attend postures) (d, e) were, respectively, recorded for 3 h (5 min per point) and for 1 h, for one single day. Significant differences between treatment and NaCl are marked (§§p<0.01).
The effects of MDMA alone on horizontal activity were significantly greater than for mice treated with MDMA plus RS102221 (MDMA vs MDMA+RS102221: +226%, p<0.01), or RS102221 (MDMA vs RS102221: +435%, p<0.01) alone (Figure 4b). After 120 min, there was no significant effect of treatment (F3,26=0.45, p=0.72, Figure 4c), time (F11,286=1.27, p=0.24, Figure 4c) or treatment × time interaction (F33,286=1.01, p=0.45, Figure 4c). In summary, MDMA stimulated locomotion in mice when injected alone, but not when coadministered with RS102221.
Stereotyped behaviors could also compete with both horizontal locomotion and feeding changes after MDMA. The absence of a reduction in the hypophagic response to MDMA when 5-HT2C receptors were inactivated may be explained by the fact that mice exhibited less stereotyped behaviors after cotreatment with MDMA plus RS102221. To investigate such a possibility, we assessed the degree of certain stereotyped behaviors. Wild-type mice treated with MDMA, RS102221, or MDMA plus RS102221 exhibited no significant difference in the number of head weaving, grooming, or licking behaviors, as compared to control animals (not shown). In contrast, both MDMA and MDMA plus RS102221 treatments induced marked increases in the number of flat body and stretch–attend postures observed, as compared to control mice, but only for the initial 30 min following drug injection (Figure 4d). No significant difference was detected after use of any treatment over the next 30 min (Figure 4e). Therefore, the recorded stereotyped behaviors may not compete with feeding or motor responses.
DISCUSSION
There are many studies reporting solid evidence that MDMA administration increases 5-HT steady-state content in several brain structures of rats and mice (McKenna and Peroutka, 1990; Colado et al, 2004). Consequently, scientists focused on the effects of the drug on serotonergic system functions, even though MDMA has varying potencies in increasing the synaptic levels of other biogenic amines, such as DA (McKenna and Peroutka, 1990; Colado et al, 2004). One dose of 10 mg/kg MDMA (i.p., 3 h), identical to that used under our experimental conditions, was found to increase 5-HT turnover in mouse brain (Steele et al, 1989). Few studies indicate that MDMA exerts an effect on food consumption in humans (anorectic or bulimic effects) (Shulgin, 1986; Rochester and Kirchner, 1999; Schifano, 2000), as would be expected from its ‘substrate-type 5-HT releaser’ activity. Furthermore, only one study reports its hypophagic effect in rats (Frith et al, 1987). Curiously, no attempt has been made to investigate the effect of MDMA on feeding behavior in mice and the possible implication of 5-HT receptors. Our results indicate that MDMA induced an initial hypophagia in spite of starvation, suggesting that the drug induced a pharmacological effect sufficient to disrupt the physiological drive in mice to eat. However, this hypophagic effect was transient (1 h) since over the following period (60–180 min) MDMA-treated mice exhibited hyperphagia compared to saline-treated mice. Therefore, the kinetics of the feeding response to MDMA was biphasic. We first thought that 5-HT1B receptors could be implicated in the effects of MDMA in starved mice. However, several points argue against this hypothesis. Firstly, we found that 5-HT1B receptor agonists (CGS12066A and RU24969) effectively reduced food consumption, but they did so differently to MDMA. Their effects were monophasic with a hypophagic response prolonged over the 180 min period of investigation. Secondly, there was no difference observed in the biphasic feeding response to MDMA on feeding behavior in starved 5-HT1B receptor-null mice vs wild-type animals. Thirdly, the 5-HT1B/1D receptor antagonist GR127935 did not modify the biphasic effect of MDMA on feeding behavior in starved mice. This result is consistent with that obtained by Vickers et al (2001), which showed that GR127935 as well as the 5-HT1A/1B receptor antagonist cyanopindolol did not reduce fenfluramine-induced anorexia in starved rats. However, 5-HT1B receptor-null mice are insensitive to fenfluramine-induced anorexia. These apparently contradictory results may be explained either by a developmental problem or, as suggested, by a reduction of 5-HT2C receptor function in 5-HT1B receptor-null mice (Clifton et al, 2003). Compensatory effects of other 5-HT receptors may occur in 5-HT1B receptor-null mice. For example, a compensatory elevation in 5-HT4 receptor function is likely because the 5-HT4 receptor antagonist RS39604 reduced MDMA-induced hypophagia (Compan et al, 2004). It remains to be determined why the 5-HT1B receptor-null mice are sensitive to MDMA-induced anorexia but not to fenfluramine-induced anorexia. Fenfluramine and MDMA have already been reported to induce different behavioral changes. MDMA administration provoked an increase in locomotion (Rempel et al, 1993) and temperature (Malberg and Seiden, 1998), whereas fenfluramine causes hypolocomotion (Bankson and Cunningham, 2001) and hypothermia (Cryan et al, 2000).
The implication of 5-HT2C receptors in MDMA-induced hypophagia is clear. The present results indicate that MDMA-induced hypophagia was greatly reduced if MDMA was coadministered with the 5-HT2C receptor antagonist RS102221. Injection of RS102221 also blocked the motor responses to MDMA, indicating that the dose used (2 mg/kg) is effective (see Bonhaus et al, 1997 for discussion). Inactivation of 5-HT2C receptors also has been reported to mediate reduced food intake following 5-HT releasers, such as fluoxetine (Lightowler et al, 1996), d-fenfluramine (Neill and Cooper, 1989; Vickers et al, 2001), and norfenfluramine (Vickers et al, 2001). Thus, MDMA acts via inducing an increase in 5-HT levels in the synaptic cleft followed by the activation of 5-HT2C receptors. A direct action of MDMA on 5-HT2C receptors cannot be excluded since MDMA does bind to 5-HT2 receptors with high affinity (0.6–6 μM) (Lyon et al, 1986; Battaglia and De Souza, 1989).
MDMA is a drug of abuse that elicits a dramatic increase in locomotion (Geyer and Callaway, 1994), suggesting the possibility that MDMA-induced hypophagia could be due to hyperlocomotion. However, this seems unlikely at least for one reason: MDMA (10 mg/kg)-induced hyperlocomotion was reduced after treatment with GR127935 and in 5-HT1B receptor-null mice (Scearce-Levie et al, 1999; Compan et al, 2003), whereas the hypophagic effects of MDMA were not. Likewise, several studies have excluded the possibility that nonspecific behavioral changes, such as hyperlocomotion and even sedation, may account for the hypophagic responses to 5-HT1B, 5-HT2A, 5-HT2B, or 5-HT2C receptor agonists (Kitchener and Dourish, 1994; Lee and Simansky, 1997; Hewitt et al, 2002; Clifton et al, 2000, 2003). The fact that 5-HT2C receptors may contribute to MDMA-induced hyperlocomotion has been questioned in one study using rats (Bankson and Cunningham, 2002). This investigation reported that SB206553, a 5-HT2C receptor antagonist, enhanced the motor effects of MDMA in the rat (Bankson and Cunningham, 2002). Such a discrepancy may likely be due to dose- or species-specific differences since an acute administration of 3.3 mg/kg MDMA did not stimulate locomotion in mice (Scearce-Levie et al, 1999), but did in rats (Bankson and Cunningham, 2002). This difference may depend on the fact that a 10 mg/kg dose of MDMA induces a marked increase in the DA release in rats, while only a modest rise is obtained at the same dose in mice (see Yamamoto et al, 1995; Colado et al, 2004). Moreover, the use of different antagonists may also lead to varied results. It has been shown recently that SB206553 is not a neutral antagonist, but an inverse agonist of 5-HT2C receptors. This compound elevated the extracellular DA levels in the nucleus accumbens of rats by a significantly greater magnitude than did the 5-HT2C receptor antagonist SB242084 (De Deurwaerdère et al, 2004). Consequently, the influence of serotonergic systems, in part by 5-HT2C receptors, appears to be dependent on the degree of elevation of DA concentration, as extensively debated by Bankson and Cunningham, in studies using rats (2001).
Our results suggest that 5-HT2C receptor transmission may underlie the initial hypophagic effect of MDMA, but that is likely not responsible for the following period of hyperphagia. Indeed, the delayed hyperphagic effect of MDMA (period 60–180 min) was not modified by the coinjection of RS102221 and MDMA. Nevertheless, this is possible that hyperphagia could be mediated via one or several other neuronal systems such as peptidergic and/or dopaminergic systems.
These findings present the first evidence that a pharmacological inactivation of 5-HT2C receptors using RS102221 overcomes both MDMA-induced feeding and motor disorders in mice.
References
Aulakh CS, Tolliver T, Wozniak KM, Hill JL, Murphy DL (1994). Functional and biochemical evidence for altered serotonergic function in the fawn-hooded rat strain. Pharmacol Biochem Behav 49: 615–620.
Bankson MG, Cunningham KA (2001). 3,4-Methylenedioxymethamphetamine (MDMA) as a unique model of serotonin receptor function and serotonin–dopamine interactions. J Pharmacol Exp Ther 297: 846–852.
Bankson MG, Cunningham KA (2002). Pharmacological studies of the acute effects of (+)-3,4-methylenedioxymethamphetamine on locomotor activity: role of 5-HT(1B/1D) and 5-HT(2) receptors. Neuropsychopharmacology 26: 40–52.
Battaglia G, De Souza EB (1989). Pharmacologic profile of amphetamine derivatives at various brain recognition sites: selective effects on serotonergic systems. NIDA Res Monogr 94: 240–258.
Bendotti C, Samanin R (1987). The role of putative 5-HT1A and 5-HT1B receptors in the control of feeding in rats. Life Sci 41: 635–642.
Bonhaus DW, Weinhardt KK, Taylor M, DeSouza A, McNeeley PM, Szczepanski K et al (1997). RS-102221: a novel high affinity and selective, 5-HT2C receptor antagonist. Neuropharmacology 36: 621–629.
Bouwknecht JA, Van der Gugten J, Hijzen TH, Maes RA, Hen R, Olivier B (2001). Male and female 5-HT1B receptor knockout mice have higher body weights than wild types. Physiol Behav 74: 507–516.
Brunner D, Buhot MC, Hen R, Hofer M (1999). Anxiety, motor activation, and maternal–infant interactions in 5HT1B knockout mice. Behav Neurosci 113: 587–601.
Clifton PG, Lee MD, Dourish CT (2000). Similarities in the action of Ro 60-0175, a 5-HT2C receptor agonist and d-fenfluramine on feeding patterns in the rat. Psychopharmacology 152: 256–267.
Clifton PG, Lee MD, Somerville EM, Kennett GA, Dourish CT (2003). 5-HT1B receptor knockout mice show a compensatory reduction in 5-HT2C receptor function. Eur J Neurosci 17: 185–190.
Colado MI, O'Shea E, Green AR (2004). Acute and long term effects of MDMA on cerebral dopamine biochemistry and function. Psychopharmacology 173: 249–263.
Compan V, Charnay Y, Dusticier N, Daszuta A, Hen R, Bockaert J (2004). Feeding disorders in 5-HT4 receptor knockout mice. J Soc Biol 198: 37–49.
Compan V, Scearce-Levie K, Crosson C, Daszuta A, Hen R (2003). Enkephalin contributes to the locomotor stimulating effects of 3,4-methylenedioxy-N-methamphetamine. Eur J Neurosci 18: 1–8.
Compan V, Zhou M, Grailhe R, Gazzara RA, Martin R, Gingrich J et al (2004). Attenuated response to stress and novelty and hypersensitivity to seizures in 5-HT4 receptor knock-out mice. J Neurosci 24: 412–419.
Cryan JF, Harkin A, Naughton M, Kelly JP, Leonard BE (2000). Characterization of dfenfluramine-induced hypothermia: evidence for multiple sites of action. Eur J Pharmacol 390: 275–285.
De Deurwaerdère P, Navailles S, Berg KA, Clarke WP, Spampinato U (2004). Constitutive activity of the serotonin 2C receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J Neurosci 24: 3235–3241.
Frith CH, Chang LW, Lattin DL, Walls RC, Hamm J, Doblin R (1987). Toxicity of methylenedioxymethamphetamine (MDMA) in the dog and the rat. Fundam Appl Toxicol 9: 110–119.
Geyer MA, Callaway CW (1994). Behavioral pharmacology of ring-substituted amphetamine analogs. In: Cho AR, Segal OS (eds). Amphetamine and its Analogs. Academic Press: New York, pp 177–201.
Halford JC, Blundell JE (1996). The 5-HT1B receptor agonist CP-94,253 reduces food intake and preserves the behavioural satiety sequence. Physiol Behav 60: 933–939.
Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL et al (2002). Activation of central melanocortin pathways by fenfluramine. Science 297: 609–611.
Hewitt KN, Lee MD, Dourish CT, Clifton PG (2002). Serotonin 2C receptor agonists and the behavioural satiety sequence in mice. Pharmacol Biochem Behav 71: 691–700.
Kennett GA, Curzon G (1988). Evidence that hypophagia induced by mCPP and TFMPP requires 5-HT1C and 5-HT1B receptors; hypophagia induced by RU 24969 only requires 5-HT1B receptors. Psychopharmacology 96: 93–100.
Kennett GA, Wood MD, Bright F, Trail B, Riley G, Holland V et al (1997). SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology 36: 609–620.
Kitchener SJ, Dourish CT (1994). An examination of the behavioural specificity of hypophagia induced by 5-HT1B, 5-HT1C and 5-HT2 receptor agonists using the post-prandial satiety sequence in rats. Psychopharmacology 113: 369–377.
Lee MD, Simansky KJ (1997). CP-94, 253: a selective serotonin1B (5-HT1B) agonist that promotes satiety. Psychopharmacology 131: 264–270.
Lee MD, Somerville EM, Kennett GA, Dourish CT, Clifton PG (2004). Reduced hypophagic effects of d-fenfluramine and the 5-HT2C receptor agonist mCPP in 5-HT1B receptor knockout mice. Psychopharmacology 176: 39–49.
Lightowler S, Wood M, Brown T, Glen A, Blackburn T, Tulloch I et al (1986). An investigation of the mechanism responsible for fluoxetine-induced hypophagia in rats. Eur J Pharmacol 296: 137–143.
Lucas J, Yamamoto A, Scearce-Levie K, Saudou F, Hen R (1998). Absence of fenfluramine-induced anorexia and reduced C-Fos induction in the hypothalamus and centre amygdaloid complex of serotonin 1B receptor knockout mice. J Neurosci 18: 5537–5544.
Lyon RA, Glennon RA, Titeler M (1986). 3,4-Methylenedioxymethamphetamine (MDMA): stereoselective interactions at brain 5-HT1 and 5-HT2 receptors. Psychopharmacology 88: 525–526.
Macor JE, Burkhart CA, Heym JH, Ives JL, Lebel LA, Newman ME et al (1990). 3-(1,2,5,6-Tetrahydropyrid-4-yl)pyrrolo[3,2-b]pyrid-5-one: a potent and selective serotonin (5-HT1B) agonist and rotationally restricted phenolic analogue of 5-methoxy-3-(1,2,5,6-tetrahydropyrid-4-yl)indole. J Med Chem 33: 2087–2093.
Malberg JE, Seiden LS (1998). Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci 18: 5086–5094.
Marona-Lewicka D, Nichols DE (1994). Behavioral effects of the highly selective serotonin releasing agent 5-methoxy-6-methyl-2-aminoindan. Eur J Pharmacol 258: 1–13.
McKenna DJ, Peroutka SJ (1990). Neurochemistry and neurotoxicity of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’). J Neurochem 54: 14–22.
Neill JC, Cooper SJ (1989). Evidence that d-fenfluramine anorexia is mediated by 5-HT1 receptors. Psychopharmacology 97: 213–218.
Nonogaki K, Strack AM, Dallman MF, Tecott LH (1998). Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat Med 4: 1152–1156.
Phillips TJ, Hen R, Crabbe JC (1999). Complications associated with genetic background effects in research using knockout mice. Psychopharmacology 147: 5–7.
Rempel NL, Callaway CW, Geyer MA (1993). Serotonin1B receptor activation mimics behavioral effects of presynaptic serotonin release. Neuropsychopharmacology 8: 201–211.
Rochester JA, Kirchner JT (1999). Ecstasy (3,4-methylenedioxymethamphetamine): history, neurochemistry, and toxicology. J Am Board Fam Pract 12: 137–142.
Rothman RB, Baumann MH (2002). Therapeutic and adverse actions of serotonin transporter substrates. Pharmacol Ther 95: 73–88.
Saudou F, Aït Amara D, Lemeur M, Ramboz S, Segu L, Buhot MC et al (1994). Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science 265: 1875–1878.
Scearce-Levie K, Viswanathan SS, Hen R (1999). Locomotor response to MDMA is attenuated in knockout mice lacking the 5-HT1B receptor. Psychopharmacology 141: 154–161.
Schechter LE, Simansky KJ (1988). 1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane (DOI) exerts an anorexic action that is blocked by 5-HT2 antagonists in rats. Psychopharmacology 94: 342–346.
Schifano F (2000). Potential human neurotoxicity of MDMA (‘ecstasy’): subjective self-reports, evidence from an Italian drug addiction centre and clinical case studies. Neuropsychobiology 42: 25–33.
Shulgin AT (1986). The background and chemistry of MDMA. J Psychoactive Drugs 18: 291–304.
Steele TD, Nichols DE, Yim GK (1989). MDMA transiently alters biogenic amines and metabolites in mouse brain and heart. Pharmacol Biochem Behav 34: 223–227.
Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF et al (1995). Eating disorder and epilepsy in mice lacking 5-HT2C serotonin receptors. Nature 374: 542–546.
Vickers SP, Clifton PG, Dourish CT, Tecott LH (1999). Reduced satiating effect of d-fenfluramine in serotonin 5-HT2C receptor mutant mice. Psychopharmacology 143: 309–314.
Vickers SP, Dourish CT, Kennett GA (2001). Evidence that hypophagia induced by d-fenfluramine and d-norfenfluramine in the rat is mediated by 5-HT2C receptors. Neuropharmacology 41: 200–209.
Yamamoto BK, Nash JF, Gudelsky GA (1995). Modulation of methylenedioxymethamphetamine-induced striatal dopamine release by the interaction between serotonin and gamma-aminobutyric acid in the substantia nigra. J Pharmacol Exp Ther 273: 1063–1070.
Acknowledgements
We greatly appreciate the assistance of A Turner-Madeuf and Dr A Brady in editing the text.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Conductier, G., Crosson, C., Hen, R. et al. 3,4-N-Methlenedioxymethamphetamine-Induced Hypophagia is Maintained in 5-HT1B Receptor Knockout Mice, but Suppressed by the 5-HT2C Receptor Antagonist RS102221. Neuropsychopharmacol 30, 1056–1063 (2005). https://doi.org/10.1038/sj.npp.1300662
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300662
Keywords
This article is cited by
-
A genetic reduction in the serotonin transporter differentially influences MDMA and heroin induced behaviours
Psychopharmacology (2018)
-
The nucleus accumbens 5-HTR4-CART pathway ties anorexia to hyperactivity
Translational Psychiatry (2012)
-
Deconstructing Antiobesity Compound Action: Requirement of Serotonin 5-HT2B Receptors for Dexfenfluramine Anorectic Effects
Neuropsychopharmacology (2011)
-
Residual social, memory and oxytocin-related changes in rats following repeated exposure to γ-hydroxybutyrate (GHB), 3,4-methylenedioxymethamphetamine (MDMA) or their combination
Psychopharmacology (2010)
-
Repeated Cocaine Administration Decreases 5-HT2A Receptor-Mediated Serotonergic Enhancement of Synaptic Activity in Rat Medial Prefrontal Cortex
Neuropsychopharmacology (2009)