Abstract
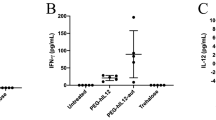
Recent studies have demonstrated that intravenous administration of a plasmid DNA–cationic liposome complex (lipoplex) induced significant proinflammatory cytokine production in blood and inhibited transgene expression in pulmonary endothelial cells. In this study, we examined the effects of gadolinium chloride (GdCl3) pretreatment on the biodistribution and induction of proinflammatory cytokine production and transgene expression after intravenous injection of a lipoplex in mice. GdCl3 is known to transiently deplete liver Kupffer cells and spleen macrophages after intravenous administration. Intravenous administration of a lipoplex triggers high levels of proinflammatory cytokine production, such as TNF-α, IFN-γ and IL-12 in serum and a large amount of 32P-labeled lipoplex accumulates in the liver 1 h after intravenous administration. However, pretreatment with GdCl3 dramatically reduces serum levels of these cytokines and liver accumulation of the lipoplex. RT-PCR analysis showed that mRNA expression of TNF-α greatly increases in the liver and spleen after lipoplex injection and that pretreatment with GdCl3 reduces mRNA expression in these organs. Messenger RNA expression of TNF-α in the liver occurs in non-parenchymal cells (sinusoidal endothelial cells and/or Kupffer cells). Inhibition of cytokine production by pretreatment with GdCl3 leads to recovery of transgene expression in the lung following the second injection of lipoplex, which was reduced following the first injection of lipoplex. Thus, the present study demonstrates that tissue macrophages involving liver Kupffer cells and spleen macrophages are closely involved in TNF-α production following i.v. administration of the lipoplex. It is also suggested that avoiding lipoplex uptake and subsequent cytokine production by these cells would be a useful method of maintaining a high level of gene expression in the lung after repeated injections.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Templeton NS et al. Improved DNA:liposome complexes for increased systemic delivery and gene expression Nature Biotechnol 1997 15: 647–652
Liu Y et al. Factors influencing the efficiency of cationic liposome-mediated intravenous gene delivery Nat Biotechol 1997 15: 167–173
Li S, Rizzo MA, Bhattacharya S, Huang L . Characterization of cationic lipid-protamine-DNA (LPD) complexes for intravenous gene delivery Gene Therapy 1998 5: 930–937
Whitmore M, Li S, Huang L . LPD lipopolyplex initiates a potent cytokine response and inhibits tumor growth Gene Therapy 1999 6: 1867–1875
Dow SW et al. Lipid-DNA complexes induced potent activation of innate immune responses and antitumor activity when administrated intravenously J Immunol 1999 163: 1552–1561
Krieg AM et al. CpG motifs in bacterial DNA trigger direct B-cell activation Nature 1995 374: 546–549
Iho S, Yamamoto T, Takahashi T, Yamamoto S . Oligodeoxynucleotides containing palindrome sequences with internal 5’-CpG-3’ act directly on human NK and activated T cells to induce IFN-γ production in vitro J Immunol 1999 163: 3642–3652
Sester DP et al. Phosphorothioate backbone modification modulates macrophage activation by CpG DNA J Immunol 2000 165: 4165–4173
Li S . Effect of immune response on gene transfer to the lung via systemic administration of cationic lipidic vectors Am J Physiol 1999 276: L796–L804
Tan Y, Li S, Pitt BR, Huang L . The inhibitory role of CpG Immunostimulatory motif in cationic lipid vector-mediated transgene expression in vivo Hum Gene Ther 1999 10: 2153–2161
Zhang Y et al. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages Mol Ther 2001 3: 697–707
Egmond MV et al. FcγRI-positive liver Kupffer cells: reappraisal of the function of immunoglobulin A in immunity Nature Med 2000 6: 680–685
Decker K . Biologically active products of stimulated liver macrophages (Kupffer cells) Eur J Biochem 1990 192: 245–261
McLean JW et al. Organ-specific endothelial cell uptake of cationic liposome-DNA complexes in mice Am J Physiol 1997 273: H384–H404
Mahato RI et al. Physicochemical and pharmacokinetic characteristics of plasmid DNA/cationic liposome complexes J Pharm Sci 1995 84: 1267–1271
Osaka G et al. Pharmacokinetics, tissue distribution, and expression efficiency of plasmid [33P]DNA following intravenous administration of DNA/cationic lipid complexes in mice: use of a novel radionucleotide approach J Pharm Sci 1996 85: 612–618
Litzinger DC et al. Fate of cationic liposomes and their complex with oligonucleotide in vivo Biochim Biophys Acta 1996 1281: 139–149
Lieber A et al. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors J Virol 1997 71: 8798–8807
Alemany R, Suzuki K, Curiel DT . Blood clearance rates of adenovirus type 5 in mice J Gen Virol 2000 81: 2605–2609
Hardonk MJ, Dijkhuis FWJ, Hulstaert CE, Koudstaal J . Heterogeneity of rat liver and spleen macrophages in gadolinium chloride-induced elimination and repopulation J Leukoc Biol 1992 52: 296–302
Husztik E, Lazar G, Parducz A . Electron microscopic study of Kupffer-cell phagocytosis blockade induced by gadolinium chloride Br J Exp Path 1980 61: 624–630
Shi J, Gilbert GE, Kokubo Y, Ohashi T . Role of the liver in regulating numbers of circulating neutophils Blood 2001 98: 1226–1230
Tsuda K et al. Eosinophil-induced liver injury: an experimental model using IL-5 transgenic mice J Hepatol 2001 34: 270–277
Yi A-K et al. CpG motifs in bacterial DNA activate leukocytes through the pH-dependent generation of reactive oxygen species J Immunol 1998 160: 4755–4761
Li S et al. Dynamic changes in the characteristics of cationic lipidic vectors after exposure to mouse serum: implications for intravenous lipofection Gene Therapy 1999 6: 585–594
Mahato RI et al. Biodistribution and gene expression of lipid/plasmid complexes after systemic administration Hum Gene Ther 1998 9: 2083–2099
Ruponen M, Yla-Herttuala S, Urrti A . Interaction of polymeric and liposomal gene delivery systems with extracellular glycosaminoglycans: physicochemical and transfection studies Biochim Biophys Acta 1999 1415: 331–341
Zelphati O et al. Effect of serum components on the physico-chemical properties of cationic lipid/oligonucleotide complexes and on their interactions with cells Biochim Biophys Acta 1998 1390: 119–133
Kawabata K, Takakura Y, Hashida M . The fate of plasmid DNA after intravenous injection in mice: involvement of scavenger receptors in its hepatic uptake Pharm Res 1995 12: 825–830
Barron LG, Gagne L, Szoka FC JR . Lipoplex-mediated gene delivery to the lung occurs within 60 minutes of intravenous administration Hum Gene Ther 1999 10: 1683–1694
Wattiaux R et al. Cationic lipids delay the transfer of plasmid DNA to lysosomes Biochem Biophys Res Commun 1996 227: 448–454
Tripp CA, Wolf SF, Unanue ER . Interleukin 12 and tumor necrosis factor α are costimulators of interferon γ production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist Proc Natl Acad Sci USA 1993 90: 3725–3729
Wang F, Wang L, Wright D, Parmely MJ . Redox imbalance differentially inhibits lipopolysaccharide-induced macrophage activation in the mouse liver Infect Immun 1999 67: 5409–5416
Black RA et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells Nature 1997 385: 729–733
Ogawara K et al. Hepatic uptake of polystyrene microspheres in rats: effect of particle size on intrahepatic distribution J Contr Rel 1999 59: 15–22
Sparwasser T et al. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells Eur J Immunol 1998 28: 2045–2054
Tsutsumi Y et al. Molecular design of hybrid tumor necrosis factor-α III: polyethylene glycol-modified tumor necrosis factor-α has markedly enhanced antitumor potency due to longer plasma half-life and higher tumor accumulation J Pharmacol Exp Ther 1996 278: 1006–1011
Keith M, Norwich KH, Wong W, Jeejeebhoy KN . The tissue distribution of tumor necrosis factor-α in rats: a compartment model Metabolism 2000 49: 1309–1317
Jackson AM, Alexandrov AB, Prescott S, James K . Production of urinary tumour necrosis factors and soluble tumor necrosis factor receptors in bladder cancer patients after bacillus Calmette-Guerin immunotherapy Cancer Immunol Immunother 1995 40: 119–124
Dechanet J et al. CD40 ligand stimulates proinflammatory cytokine production by human endothelial cells J Immunol 1997 159: 5640–5647
Petrache I . Differential effect of MLC kinase in TNF-α-induced endothelial cell apoptosis and barrier dysfunction Am J Physiol 2001 280: L1168–L1178
Bulotta S et al. Activation of the endothelial nitric-oxide synthase by tumor necrosis factor-α J Biol Chem 2001 276: 6529–6536
Ghazizadeh SJ, Carroll JM, Taichman LB . Repression of retrovirus-mediated transgene expression by interferon: implications for gene therapy J Virol 1997 71: 9163–9369
Qin L et al. Promoter attenuation in gene therapy: interferon-gamma and tumor-necrosis factor-alpha inhibit transgene expression Hum Gene Ther 1997 8: 2019–2029
Li S, Huang L . In vivo gene transfer via intravenous administration of cationic lipid-protamine-DNA (LPD) complexes Gene Therapy 1997 4: 891–900
Song YK, Liu F, Chu SY, Liu DX . Characterization of cationic liposome-mediated gene transfer in vivo by intravenous administration Hum Gene Ther 1997 8: 1585–1594
Yoshidome H, Atsushi K, Edwards MJ, Lentsch AB . Interleukin-10 suppresses hepatic ischemia/reperfusion injury in mice: implications of a central role for nuclear factor κB Hepatology 1999 30: 203–208
Kawakami S et al. Mannose receptor-mediated gene transfer into macrophages using novel mannosylated cationic liposomes Gene Therapy 2000 7: 292–299
Acknowledgements
This work was supported in part by a Grant-In-Aid for Scientific Research from the Ministry of Education, Science and Culture, Japan.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sakurai, F., Terada, T., Yasuda, K. et al. The role of tissue macrophages in the induction of proinflammatory cytokine production following intravenous injection of lipoplexes. Gene Ther 9, 1120–1126 (2002). https://doi.org/10.1038/sj.gt.3301784
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301784
Keywords
This article is cited by
-
Nanoparticle Delivery Systems in Cancer Vaccines
Pharmaceutical Research (2011)
-
TLR9 and IRF3 Cooperate to Induce a Systemic Inflammatory Response in Mice Injected With Liposome:DNA
Molecular Therapy (2010)
-
Enhanced growth inhibition of metastatic lung tumors by intravenous injection of ATRA-cationic liposome/IL-12 pDNA complexes in mice
Cancer Gene Therapy (2010)
-
Several Serum Proteins Significantly Decrease Inflammatory Response to Lipid-based Non-viral Vectors
Molecular Therapy (2008)
-
Systemic Delivery of DNA or siRNA Mediated by Linear Polyethylenimine (L-PEI) Does Not Induce an Inflammatory Response
Pharmaceutical Research (2008)