Key Points
-
Type I interferon (IFN) responses are regulated by host, pathogen and environmental factors. These factors calibrate the host defences while limiting tissue damage and preventing autoimmunity.
-
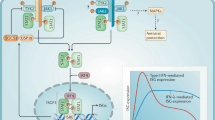
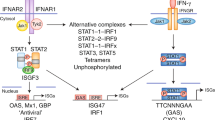
Type I IFNs signal via the IFNα receptor (IFNAR) to activate receptor-associated Janus kinase 1 (JAK1) and tyrosine kinase 2 (TYK2) kinases and downstream signal transducer and activator of transcription (STAT) transcription factors; these transcription factors then induce the expression of IFN-stimulated genes (ISGs). Type I IFNs activate the IFN-stimulated gene factor 3 (ISGF3) complex, which is comprised of STAT1, STAT2 and IFN-regulatory factor 9 (IRF9); the ISGF3 complex binds to IFN-stimulated response elements (ISREs) to induce the expression of antiviral genes.
-
Type I IFN signalling is regulated in a quantitative and qualitative manner. The magnitude of signalling is increased by induction of STAT1 and IRF9 expression and amplification of JAK signalling by spleen tyrosine kinase (SYK) and protein tyrosine kinase 2 (PYK2). Conversely, the magnitude of signalling is decreased by suppressor of cytokine signalling (SOCS) and ubiquitin carboxy-terminal hydrolase 18 (USP18) proteins and by the downregulation and internalization of IFNAR. The qualitative nature of type I IFN responses is determined by the balance between the activation of various STATs and ISGF3.
-
Type I IFN-induced transcription is regulated by the post-translational modification of STATs, chromatin remodelling, the epigenetic landscape and cooperation with other transcription factors, co-activators and co-repressors.
-
ISGs encode proteins that regulate the translation of IFNAR and JAK–STAT signalling components and of ISGs themselves. Type I IFNs also induce the expression of microRNAs that regulate the IFN response.
-
Chronic type I IFN responses can promote autoimmunity by increasing antigen presentation, lymphocyte-mediated adaptive immune responses and chemokine expression. In chronic infections, type I IFNs can induce immunosuppression in part by increasing expression of interleukin-10 and programmed cell death 1 ligand 1 (PDL1).
Abstract
Type I interferons (IFNs) activate intracellular antimicrobial programmes and influence the development of innate and adaptive immune responses. Canonical type I IFN signalling activates the Janus kinase (JAK)–signal transducer and activator of transcription (STAT) pathway, leading to transcription of IFN-stimulated genes (ISGs). Host, pathogen and environmental factors regulate the responses of cells to this signalling pathway and thus calibrate host defences while limiting tissue damage and preventing autoimmunity. Here, we summarize the signalling and epigenetic mechanisms that regulate type I IFN-induced STAT activation and ISG transcription and translation. These regulatory mechanisms determine the biological outcomes of type I IFN responses and whether pathogens are cleared effectively or chronic infection or autoimmune disease ensues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Trinchieri, G. Type I interferon: friend or foe? J. Exp. Med. 207, 2053–2063 (2010).
Pestka, S., Krause, C. D. & Walter, M. R. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 202, 8–32 (2004).
Hertzog, P. J. & Williams, B. R. Fine tuning type I interferon responses. Cytokine Growth Factor Rev. 24, 217–225 (2013).
Paludan, S. R. & Bowie, A. G. Immune sensing of DNA. Immunity 38, 870–880 (2013).
Goubau, D., Deddouche, S. & Reis, E. S. C. Cytosolic sensing of viruses. Immunity 38, 855–869 (2013).
Iwasaki, A. A virological view of innate immune recognition. Annu. Rev. Microbiol. 66, 177–196 (2012).
Levy, D. E. & Darnell, J. E. Jr. STATs: transcriptional control and biological impact. Nature Rev. Mol. Cell Biol. 3, 651–662 (2002).
Stark, G. R. & Darnell, J. E. Jr. The JAK-STAT pathway at twenty. Immunity 36, 503–514 (2012).
MacMicking, J. D. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nature Rev. Immunol. 12, 367–382 (2012).
Schoggins, J. W. et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472, 481–485 (2011). This study identifies specific antiviral functions for multiple ISGs, showing that unique sets of ISGs target distinct viruses. It highlights the importance of translational regulation.
Rusinova, I. et al. Interferome v2.0: an updated database of annotated interferon-regulated genes. Nucleic Acids Res. 41, D1040–D1046 (2012).
Saka, H. A. & Valdivia, R. Emerging roles for lipid droplets in immunity and host-pathogen interactions. Annu. Rev. Cell Dev. Biol. 28, 411–437 (2012).
van Boxel-Dezaire, A. H., Rani, M. R. & Stark, G. R. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity 25, 361–372 (2006).
Gough, D. J., Messina, N. L., Clarke, C. J., Johnstone, R. W. & Levy, D. E. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity 36, 166–174 (2012).
Abt, M. C. et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 37, 158–170 (2012). Together with references 16 and 17, this study demonstrates that the commensal microflora calibrates innate immune responses and maintains homeostasis in part by providing tonic signals that maintain a basal systemic IFN response.
Ganal, S. C. et al. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity 37, 171–186 (2012).
Kawashima, T. et al. Double-stranded RNA of intestinal commensal but not pathogenic bacteria triggers production of protective interferon-β. Immunity 38, 1187–1197 (2013).
Wang, L. et al. 'Tuning' of type I interferon-induced Jak-STAT1 signaling by calcium-dependent kinases in macrophages. Nature Immunol. 9, 186–193 (2008). This study demonstrates cross-regulation of type I IFN signalling by ITAM-associated receptors, with resultant fine-tuning of ISG induction.
Gilchrist, D. A. et al. Regulating the regulators: the pervasive effects of Pol II pausing on stimulus-responsive gene networks. Genes Dev. 26, 933–944 (2012).
Papadopoulou, A. S. et al. The thymic epithelial microRNA network elevates the threshold for infection-associated thymic involution via miR-29a mediated suppression of the IFN-α receptor. Nature Immunol. 13, 181–187 (2012).
Lu, L. F. et al. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell 142, 914–929 (2010).
Gracias, D. T. et al. The microRNA miR-155 controls CD8+ T cell responses by regulating interferon signaling. Nature Immunol. 14, 593–602 (2013). This study establishes that miRNAs regulate type I IFN responses by demonstrating that miR-155 targets IFN signalling components.
David, M. Interferons and microRNAs. J. Interferon Cytokine Res. 30, 825–828 (2010).
Levy, D. E., Lew, D. J., Decker, T., Kessler, D. S. & Darnell, J. E. Jr. Synergistic interaction between interferon-α and interferon-γ through induced synthesis of one subunit of the transcription factor ISGF3. EMBO J. 9, 1105–1111 (1990). Together with reference 25, this study demonstrates that the priming of augmented IFN responses is mediated by increased expression of IRF9 and STAT1.
Hu, X. et al. Sensitization of IFN-γ Jak-STAT signaling during macrophage activation. Nature Immunol. 3, 859–866 (2002).
Tassiulas, I. et al. Amplification of IFN-α-induced STAT1 activation and inflammatory function by Syk and ITAM-containing adaptors. Nature Immunol. 5, 1181–1189 (2004).
Hu, X., Park-Min, K. H., Ho, H. H. & Ivashkiv, L. B. IFN-γ-primed macrophages exhibit increased CCR2-dependent migration and altered IFN-γ responses mediated by Stat1. J. Immunol. 175, 3637–3647 (2005).
Yarilina, A., Park-Min, K.-H., Antoniv, T., Hu, X. & Ivashkiv, L. B. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon-response genes. Nature Immunol. 9, 378–387 (2008).
Venkatesh, D. et al. Endothelial TNF receptor 2 induces IRF1 transcription factor-dependent interferon-beta autocrine signaling to promote monocyte recruitment. Immunity 38, 1025–1037 (2013).
Cheon, H. et al. IFNβ-dependent increases in STAT1, STAT2, and IRF9 mediate resistance to viruses and DNA damage. EMBO J. 32, 2751–2763 (2013).
Fuchs, S. Y. Hope and fear for interferon: the receptor-centric outlook on the future of interferon therapy. J. Interferon Cytokine Res. 33, 211–225 (2013).
de Weerd, N. A. & Nguyen, T. The interferons and their receptors--distribution and regulation. Immunol. Cell Biol. 90, 483–491 (2012).
Bhattacharya, S. et al. Anti-tumorigenic effects of Type 1 interferon are subdued by integrated stress responses. Oncogene 32, 4214–4221 (2013).
Huynh, L., Wang, L., Shi, C., Park-Min, K. H. & Ivashkiv, L. B. ITAM-coupled receptors inhibit IFNAR signaling and alter macrophage responses to TLR4 and Listeria monocytogenes. J. Immunol. 188, 3447–3457 (2012).
Huangfu, W.-C. et al. Inflammatory signaling compromises cell responses to interferon alpha. Oncogene 31, 161–172 (2011).
Liu, J. et al. Virus-induced unfolded protein response attenuates antiviral defenses via phosphorylation-dependent degradation of the type I interferon receptor. Cell Host Microbe 5, 72–83 (2009).
Wang, L. et al. Indirect inhibition of Toll-like receptor and type I interferon responses by ITAM-coupled receptors and integrins. Immunity 32, 518–530 (2010).
Du, Z. et al. Inhibition of IFN-α signaling by a PKC- and protein tyrosine phosphatase SHP-2-dependent pathway. Proc. Natl Acad. Sci. USA 102, 10267–10272 (2005).
Yoshimura, A., Naka, T. & Kubo, M. SOCS proteins, cytokine signalling and immune regulation. Nature Rev. Immunol. 7, 454–465 (2007).
Sarasin-Filipowicz, M. et al. Alpha interferon induces long-lasting refractoriness of JAK-STAT signaling in the mouse liver through induction of USP18/UBP43. Mol. Cell. Biol. 29, 4841–4851 (2009).
Nazarov, P. V. et al. Interplay of microRNAs, transcription factors and target genes: linking dynamic expression changes to function. Nucleic Acids Res. 41, 2817–2831 (2013).
Tili, E. et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-α stimulation and their possible roles in regulating the response to endotoxin shock. J. Immunol. 179, 5082–5089 (2007).
Murray, P. J. The JAK-STAT signaling pathway: input and output integration. J. Immunol. 178, 2623–2629 (2007).
Ho, H. H. & Ivashkiv, L. B. Role of STAT3 in type I interferon responses. Negative regulation of STAT1-dependent inflammatory gene activation. J. Biol. Chem. 281, 14111–14118 (2006).
Wang, W. B., Levy, D. E. & Lee, C. K. STAT3 negatively regulates type I IFN-mediated antiviral response. J. Immunol. 187, 2578–2585 (2011).
Nguyen, K. B. et al. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science 297, 2063–2066 (2002). This study demonstrates a switch in type I IFN signalling from STAT1 to STAT4 that shapes CD8+ T cell responses during viral infection in vivo.
Gil, M. P. et al. Regulating type 1 IFN effects in CD8 T cells during viral infections: changing STAT4 and STAT1 expression for function. Blood 120, 3718–3728 (2012).
Tenoever, B. R. et al. Multiple functions of the IKK-related kinase IKKε in interferon-mediated antiviral immunity. Science 315, 1274–1278 (2007).
Ng, S. L. et al. IkappaB kinase epsilon (IKKε) regulates the balance between type I and type II interferon responses. Proc. Natl Acad. Sci. USA 108, 21170–21175 (2012).
Robinson, N. et al. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nature Immunol. 13, 954–962 (2012).
de Weerd, N. A. et al. Structural basis of a unique interferon-β signaling axis mediated via the receptor IFNAR1. Nature Immunol. 14, 901–907 (2013).
Sadzak, I. et al. Recruitment of Stat1 to chromatin is required for interferon-induced serine phosphorylation of Stat1 transactivation domain. Proc. Natl Acad. Sci. USA 105, 8944–8949 (2008).
Bancerek, J. et al. CDK8 kinase phosphorylates transcription factor STAT1 to selectively regulate the interferon response. Immunity 38, 250–262 (2013).
Yang, J. et al. Reversible methylation of promoter-bound STAT3 by histone-modifying enzymes. Proc. Natl Acad. Sci. USA 107, 21499–21504 (2010).
Droescher, M., Begitt, A., Marg, A., Zacharias, M. & Vinkemeier, U. Cytokine-induced paracrystals prolong the activity of signal transducers and activators of transcription (STAT) and provide a model for the regulation of protein solubility by small ubiquitin-like modifier (SUMO). J. Biol. Chem. 286, 18731–18746 (2011).
Hu, X. & Ivashkiv, L. B. Cross-regulation of signaling pathways by interferon-γ: implications for immune responses and autoimmune diseases. Immunity 31, 539–550 (2009).
Tamura, T., Yanai, H., Savitsky, D. & Taniguchi, T. The IRF family transcription factors in immunity and oncogenesis. Annu. Rev. Immunol. 26, 535–584 (2008).
Levy, D. E., Marie, I., Smith, E. & Prakash, A. Enhancement and diversification of IFN induction by IRF-7-mediated positive feedback. J. Interferon Cytokine Res. 22, 87–93 (2002).
Farlik, M. et al. Contribution of a TANK-binding kinase 1-interferon (IFN) regulatory factor 7 pathway to IFN-γ-induced gene expression. Mol. Cell. Biol. 32, 1032–1043 (2012).
Qiao, Y. et al. Synergistic activation of inflammatory cytokine genes by interferon-γ-induced chromatin remodeling and Toll-like receptor signaling. Immunity 39, 454–469 (2013). Together with references 79 and 80, this study demonstrates a pervasive binding of STAT1 to promoters and enhancers genome-wide that programs cellular responses to environmental cues.
Chatterjee-Kishore, M., Wright, K. L., Ting, J. P. & Stark, G. R. How Stat1 mediates constitutive gene expression: a complex of unphosphorylated Stat1 and IRF1 supports transcription of the LMP2 gene. EMBO J. 19, 4111–4122 (2000).
Farlik, M. et al. Nonconventional initiation complex assembly by STAT and NF-κB transcription factors regulates nitric oxide synthase expression. Immunity 33, 25–34 (2010).
Xu, D. et al. Promyelocytic leukemia zinc finger protein regulates interferon-mediated innate immunity. Immunity 30, 802–816 (2009).
Litvak, V. et al. A FOXO3-IRF7 gene regulatory circuit limits inflammatory sequelae of antiviral responses. Nature 490, 421–425 (2012).
Shalek, A. K. et al. Single-cell transcriptomics reveals bimodality in expression and splicing in immune cells. Nature 498, 236–240 (2013).
Zhao, M., Zhang, J., Phatnani, H., Scheu, S. & Maniatis, T. Stochastic expression of the interferon-beta gene. PLoS Biol. 10, e1001249 (2012).
Hwang, S. Y. et al. Biphasic RLR-IFN-β response controls the balance between antiviral immunity and cell damage. J. Immunol. 190, 1192–1200 (2013).
Bell, O., Tiwari, V. K., Thoma, N. H. & Schubeler, D. Determinants and dynamics of genome accessibility. Nature Rev. Genet. 12, 554–564 (2011).
Smale, S. T. Selective transcription in response to an inflammatory stimulus. Cell 140, 833–844 (2010).
Yan, Z. et al. PBAF chromatin-remodeling complex requires a novel specificity subunit, BAF200, to regulate expression of selective interferon-responsive genes. Genes Dev. 19, 1662–1667 (2005).
Liu, H., Kang, H., Liu, R., Chen, X. & Zhao, K. Maximal induction of a subset of interferon target genes requires the chromatin-remodeling activity of the BAF complex. Mol. Cell. Biol. 22, 6471–6479 (2002).
Cui, K. et al. The chromatin-remodeling BAF complex mediates cellular antiviral activities by promoter priming. Mol. Cell. Biol. 24, 4476–4486 (2004).
Huang, M. et al. Chromatin-remodelling factor BRG1 selectively activates a subset of interferon-α-inducible genes. Nature Cell Biol. 4, 774–781 (2002).
Ni, Z. et al. Apical role for BRG1 in cytokine-induced promoter assembly. Proc. Natl Acad. Sci. USA 102, 14611–14616 (2005).
Ramirez-Carrozzi, V. R. et al. Selective and antagonistic functions of SWI/SNF and Mi-2β nucleosome remodeling complexes during an inflammatory response. Genes Dev. 20, 282–296 (2006). This study establishes the importance of chromatin remodelling for the induction of inflammatory genes and ISGs.
Ramirez-Carrozzi, V. R. et al. A unifying model for the selective regulation of inducible transcription by CpG islands and nucleosome remodeling. Cell 138, 114–128 (2009).
Bhatt, D. M. et al. Transcript dynamics of proinflammatory genes revealed by sequence analysis of subcellular RNA fractions. Cell 150, 279–290 (2012).
Zentner, G. E. & Henikoff, S. Regulation of nucleosome dynamics by histone modifications. Nature Struct. Mol. Biol. 20, 259–266 (2013).
Vahedi, G. et al. STATs shape the active enhancer landscape of T cell populations. Cell 151, 981–993 (2012).
Ostuni, R. et al. Latent enhancers activated by stimulation in differentiated cells. Cell 152, 157–171 (2013).
Hargreaves, D. C., Horng, T. & Medzhitov, R. Control of inducible gene expression by signal-dependent transcriptional elongation. Cell 138, 129–145 (2009).
Nicodeme, E. et al. Suppression of inflammation by a synthetic histone mimic. Nature 468, 1119–1123 (2010). This study demonstrates the feasibility of therapeutic targeting of chromatin regulatory proteins to selectively suppress inflammatory gene and ISG expression.
Patel, M. C. et al. BRD4 coordinates recruitment of pause release factor P-TEFb and the pausing complex NELF/DSIF to regulate transcription elongation of interferon-stimulated genes. Mol. Cell. Biol. 33, 2497–2507 (2013).
Shakespear, M. R., Halili, M. A., Irvine, K. M., Fairlie, D. P. & Sweet, M. J. Histone deacetylases as regulators of inflammation and immunity. Trends Immunol. 32, 335–343 (2011).
Fonseca, G. J. et al. Adenovirus evasion of interferon-mediated innate immunity by direct antagonism of a cellular histone posttranslational modification. Cell Host Microbe 11, 597–606 (2012).
Lau, J. F., Nusinzon, I., Burakov, D., Freedman, L. P. & Horvath, C. M. Role of metazoan mediator proteins in interferon-responsive transcription. Mol. Cell. Biol. 23, 620–628 (2003).
Gnatovskiy, L., Mita, P. & Levy, D. E. The human RVB complex is required for efficient transcription of type I IFN-stimulated genes. Mol Cell. Biol. 33, 3817–3825 (2013).
Flammer, J. R. et al. The type I interferon signaling pathway is a target for glucocorticoid inhibition. Mol. Cell. Biol. 30, 4564–4574 (2010).
Icardi, L. et al. The Sin3a repressor complex is a master regulator of STAT transcriptional activity. Proc. Natl Acad. Sci. USA 109, 12058–12063 (2012). This study reveals the association of co-repressors with specific STATs as a mechanism that can selectively silence the expression of subsets of type I IFN response genes.
Tahk, S. et al. Control of specificity and magnitude of NF-κB and STAT1-mediated gene activation through PIASy and PIAS1 cooperation. Proc. Natl Acad. Sci. USA 104, 11643–11648 (2007).
Shuai, K. & Liu, B. Regulation of gene-activation pathways by PIAS proteins in the immune system. Nature Rev. Immunol. 5, 593–605 (2005).
Liu, B., Tahk, S., Yee, K. M., Fan, G. & Shuai, K. The ligase PIAS1 restricts natural regulatory T cell differentiation by epigenetic repression. Science 330, 521–525 (2010).
Marazzi, I. et al. Suppression of the antiviral response by an influenza histone mimic. Nature 483, 428–433 (2012).
Natoli, G., Ghisletti, S. & Barozzi, I. The genomic landscapes of inflammation. Genes Dev. 25, 101–106 (2011).
Fang, T. C. et al. Histone H3 lysine 9 di-methylation as an epigenetic signature of the interferon response. J. Exp. Med. 209, 661–669 (2012).
Ea, C. K., Hao, S., Yeo, K. S. & Baltimore, D. EHMT1 protein binds to nuclear factor-κB p50 and represses gene expression. J. Biol. Chem. 287, 31207–31217 (2012).
Walsh, D., Mathews, M. B. & Mohr, I. Tinkering with translation: protein synthesis in virus-infected cells. Cold Spring Harb. Perspect. Biol. 5, a012351 (2013).
Kaur, S. et al. Regulatory effects of mTORC2 complexes in type I IFN signaling and in the generation of IFN responses. Proc. Natl Acad. Sci. USA 109, 7723–7728 (2012).
Kaur, S. et al. Role of the Akt pathway in mRNA translation of interferon-stimulated genes. Proc. Natl Acad. Sci. USA 105, 4808–4813 (2008).
Joshi, S., Kaur, S., Kroczynska, B. & Platanias, L. C. Mechanisms of mRNA translation of interferon stimulated genes. Cytokine 52, 123–127 (2010).
Rani, M. R., Hibbert, L., Sizemore, N., Stark, G. R. & Ransohoff, R. M. Requirement of phosphoinositide 3-kinase and Akt for interferon-β-mediated induction of the beta-R1 (SCYB11) gene. J. Biol. Chem. 277, 38456–38461 (2002).
Thoreen, C. C. et al. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 485, 109–113 (2012).
Hsieh, A. C. et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature 485, 55–61 (2012).
Colina, R. et al. Translational control of the innate immune response through IRF-7. Nature 452, 323–328 (2008).
Ruggieri, A. et al. Dynamic oscillation of translation and stress granule formation mark the cellular response to virus infection. Cell Host Microbe 12, 71–85 (2012).
Terenzi, F., Hui, D. J., Merrick, W. C. & Sen, G. C. Distinct induction patterns and functions of two closely related interferon-inducible human genes, ISG54 and ISG56. J. Biol. Chem. 281, 34064–34071 (2006).
Fensterl, V. & Sen, G. C. The ISG56/IFIT1 gene family. J. Interferon Cytokine Res. 31, 71–78 (2011).
Lee, M. S., Kim, B., Oh, G. T. & Kim, Y. J. OASL1 inhibits translation of the type I interferon-regulating transcription factor IRF7. Nature Immunol. 14, 346–355 (2013).
Hall, J. C. & Rosen, A. Type I interferons: crucial participants in disease amplification in autoimmunity. Nature Rev. Rheumatol. 6, 40–49 (2010).
Maurano, M. T. et al. Systematic localization of common disease-associated variation in regulatory DNA. Science 337, 1190–1195 (2012).
Rai, E. & Wakeland, E. K. Genetic predisposition to autoimmunity—what have we learned? Semin. Immunol. 23, 67–83 (2011).
Forster, S. Interferon signatures in immune disorders and disease. Immunol. Cell Biol. 90, 520–527 (2012).
Teles, R. M. B. et al. Type I interferon suppresses type II interferon-triggered human anti-mycobacterial responses. Science 339, 1448–1453 (2013). Together with references 114–116 and 124–127, this study demonstrates a dominant suppressive function of type I IFNs in chronic infections that is mediated by the induction of IL-10 and PDL1.
Berry, M. P. et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466, 973–977 (2010).
McNab, F. W. et al. TPL-2-ERK1/2 signaling promotes host resistance against intracellular bacterial infection by negative regulation of type I IFN production. J. Immunol. 191, 1732–1743 (2013).
O'Garra, A. et al. The immune response in tuberculosis. Annu. Rev. Immunol. 31, 475–527 (2013).
Kalliolias, G. D. & Ivashkiv, L. B. Overview of the biology of type I interferons. Arthritis Res. Ther. 12 (Suppl. 1), S1 (2010).
Ivashkiv, L. B. PTPN22 in autoimmunity: different cell and different way. Immunity 39, 91–93 (2013).
Wang, Y. et al. The autoimmunity-associated gene PTPN22 potentiates toll-like receptor-driven, type 1 interferon-dependent immunity. Immunity 39, 111–122 (2013).
Prinz, M. et al. Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity 28, 675–686 (2008).
Cantaert, T., Baeten, D., Tak, P. P. & van Baarsen, L. G. Type I IFN and TNFalpha cross-regulation in immune- mediated inflammatory disease: basic concepts and clinical relevance. Arthritis Res. Ther. 12, 219 (2010).
Banchereau, J., Pascual, V. & Palucka, A. K. Autoimmunity through cytokine-induced dendritic cell activation. Immunity 20, 539–550 (2004).
Gordon, R. A., Grigoriev, G., Lee, A., Kalliolias, G. D. & Ivashkiv, L. B. The interferon signature and STAT1 expression in rheumatoid arthritis synovial fluid macrophages are induced by tumor necrosis factor alpha and counter-regulated by the synovial fluid microenvironment. Arthritis Rheum. 64, 3119–3128 (2012).
Wilson, E. B. et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 340, 202–207 (2013).
Teijaro, J. R. et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 340, 207–211 (2013).
Antonelli, L. R. et al. Intranasal Poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J. Clin. Invest. 120, 1674–1682 (2010).
Mayer-Barber, K. D. et al. Innate and adaptive interferons suppress IL-1α and IL-1β production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity 35, 1023–1034 (2011).
Su, A. I. et al. Genomic analysis of the host response to hepatitis C virus infection. Proc. Natl Acad. Sci. USA 99, 15669–15674 (2002).
Guidotti, L. G. & Chisari, F. V. Immunobiology and pathogenesis of viral hepatitis. Annu. Rev. Pathol. 1, 23–61 (2006).
Kulpa, D. A. et al. PD-1 coinhibitory signals: the link between pathogenesis and protection. Semin. Immunol. 25, 219–227 (2013).
Hajishengallis, G. & Lambris, J. D. Microbial manipulation of receptor crosstalk in innate immunity. Nature Rev. Immunol. 11, 187–200 (2011).
Maecker, H. T., McCoy, J. P. & Nussenblatt, R. Standardizing immunophenotyping for the Human Immunology Project. Nature Rev. Immunol. 12, 191–200 (2012).
Davis, M. M. Immunology taught by humans. Sci Transl. Med. 4, 117fs2 (2012).
Fung, K. Y. et al. Interferon-ε protects the female reproductive tract from viral and bacterial infection. Science 339, 1088–1092 (2013).
Wang, H. et al. The role of glycogen synthase kinase 3 in regulating IFN-β-mediated IL-10 production. J. Immunol. 186, 675–684 (2011).
Chen, X. et al. Requirement for the histone deacetylase Hdac3 for the inflammatory gene expression program in macrophages. Proc. Natl Acad. Sci. USA 109, E2865–E2874 (2012).
Khabar, K. S. & Young, H. A. Post-transcriptional control of the interferon system. Biochimie 89, 761–769 (2007).
Schulz, O. et al. Protein kinase R contributes to immunity against specific viruses by regulating interferon mRNA integrity. Cell Host Microbe 7, 354–361 (2010).
Acknowledgements
We thank P. Crow for critical review of the manuscript. This work was supported by grants from the US National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- MicroRNAs
-
(miRNAs). Small RNA molecules that regulate the expression of genes by binding to the 3′ untranslated regions of specific mRNAs.
- Degron sequence
-
Degrons are signals within proteins that target them for rapid degradation. Degrons can be overt, as in the case of the N-end rule, or covert, as in the case of cyclins. For example, cyclin B must be rapidly destroyed after mitosis, and this is achieved by kinase-regulated access to a 'destruction box' sequence in cyclin B that stimulates polyubiquitylation and subsequent degradation by the proteasome.
- Mediator
-
A multiprotein complex that functions as a co-activator of transcription in all eukaryotes.
- Sumoylated
-
A sumoylated protein has undergone a type of post-translational protein modification in which the ubiquitin-like protein SUMO (small ubiquitin-related modifier) is covalently attached to the protein by an enzymatic cascade that is analogous to the cascade involved in protein ubiquitylation.
- CpG islands
-
Sequences of 0.5–2 kilobases that are rich in CpG dinucleotides. They are mostly located upstream of housekeeping genes and also of some tissue-specific genes. They are constitutively hypomethylated in many animal cell types.
- RVB proteins
-
ATP-binding proteins that belong to the ATPase- associated with diverse cellular activities (AAA) family of ATPases. They are found in different protein and nucleoprotein complexes that have roles in diverse cellular responses, including transcription, mitosis, development, apoptosis and DNA damage responses.
- IFN signature
-
A pattern of increased expression of interferon- stimulated genes (ISGs) in tissue samples or stimulated cells. The IFN signature is typically detected by using a high-throughput approach, such as microarray or RNA sequencing, to analyse gene expression.
Rights and permissions
About this article
Cite this article
Ivashkiv, L., Donlin, L. Regulation of type I interferon responses. Nat Rev Immunol 14, 36–49 (2014). https://doi.org/10.1038/nri3581
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3581
This article is cited by
-
Role of sex in immune response and epigenetic mechanisms
Epigenetics & Chromatin (2024)
-
The helicase domain of human Dicer prevents RNAi-independent activation of antiviral and inflammatory pathways
The EMBO Journal (2024)
-
Role of MDA5 Deficiency in Pathogenesis of Fungal Infections
Indian Journal of Pediatrics (2024)
-
Personalizing Oncolytic Immunovirotherapy Approaches
Molecular Diagnosis & Therapy (2024)
-
Evolutionary and functional conservation of IRF7 in the Tibetan frog Nanorana parkeri
Molecular Biology Reports (2024)