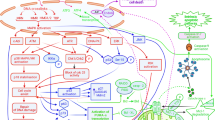
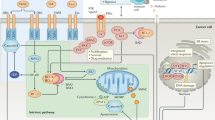
Key Points
-
Many anticancer agents induce nuclear factor-κB (NF-κB) nuclear translocation and activation of its target genes, which impinge on cellular resistance to anticancer agents.
-
Many malignant tumours display constitutive NF-κB activation that allows malignant cells to escape apoptosis.
-
NF-κB inhibition prevents tumour resistance to chemotherapeutic agents, so development of NF-κB inhibitors could increase the efficacy of many anticancer agents.
-
Several NF-κB inhibitors, such as proteasome inhibitors, BAY 11-7085, BAY 11-7082, soy isoflavone genistein, parthenolide, CHS 828, flavopiridol and gliotoxin, enhance the cytotoxic effect of anticancer agents.
-
It has also been demonstrated that steroids and non-steroidal anti-inflammatory drugs, such as cyclooxygenase-2 inhibitors, block NF-κB activation.
Abstract
The cytotoxicity of chemotherapeutic agents is attributed to apoptosis. Acquired resistance to the effects of chemotherapy has emerged as a significant impediment to effective cancer therapy. One feature that cytotoxic treatments of cancer have in common is their activation of the transcription factor nuclear factor-κB (NF-κB), which regulates cell survival. NF-κB activation suppresses the apoptotic potential of chemotherapeutic agents and contributes to resistance. What evidence is there that inhibitors of NF-κB might promote apoptosis in cancer cells and can NF-κB inhibitors be used to overcome resistance to chemotherapeutic agents?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bonizzi, G. & Karin, M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 25, 280–288 (2004). An useful summary of the NF-κB pathways in the immune response.
Jobin, C. & Sartor, R. B. The IκB/NF-κB system: a key determinant of mucosa inflammation and protection. Am. J. Physiol. Cell Physiol. 278, C451–C562 (2000).
Senftleben, U. et al. Activation by IKKα of a second, evolutionary conserved, NF-κB signaling pathway. Science 293, 1495–1499 (2001).
Hayden, M, S. & Ghosh, S. Signaling to NF-κB. Gene. Dev. 18, 2195–2224 (2004).
Brummelkamp, T. R., Nijman, S. M. B., Dirac, A. M. G. & Bernards, R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-κB. Nature 424, 797–801 (2003).
Garkavtsev, I. et al. The candidate tumour suppressor protein ING4 regulates brain tumour growth and angiogensis. Nature 428, 328–332 (2004).
Pikarsky, E. et al. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature 431, 461–466 (2004). Presents evidence that the survival of hepatocytes and their progression to malignancy are regulated by NF-κB.
Greten, F. R. et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118, 285–296 (2004).
Bottero, V. et al. Activation of nuclear factor-κB through the IKK complex by the topoisomerase poisons SN38 and doxorubicin: a brake to apoptosis in HeLa human carcinoma cells. Cancer Res. 61, 7785–7791 (2001).
Bourgarel-Rey, V. et al. Opposite effects of antimicrotubule agents on c-myc oncogene expression depending on the cell lines used. Eur. J. Cancer 36, 1043–1049 (2000).
Ji, L., Arcinas, M. & Boxer, L. M. NF-κB sites function as positive regulators of expression of the translocated c-myc allele in Burkitt's lymphoma. Mol. Cell. Biol. 14, 7967–7974 (1994).
Bourgarel-Rey, V. et al. Involvement of nuclear factor κB in c-myc induction by tubulin polymerization inhibitors. Mol. Pharmacol. 59, 1165–1170 (2001).
Ozes, O. N. et al. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 401, 82–85 (1999).
Tergaonkar, V., Pando, M., Vafa, O., Wahl, G., & Verma, I. p53 stabilization is decreased upon NFκB activation: a role for NFκB in acquisition of resistance to chemotherapy. Cancer Cell 1, 493–503 (2002).
Patel, N. M. et al. Paclitaxel sensitivity of breast cancer cells with constitutively active NF-κB is enhanced by IκBα super-repressor and parthenoide. Oncogene 19, 4159–4169 (2000).
Arlt, A. et al. Inhibition of NF-κB sensitizes human pancreatic carcinoma cells to apoptosis induced by etoposide (VP16) or doxorubicin. Oncogene 20, 859–868 (2001).
Mabuchi, S. et al. Inhibition of NFκB increases the efficacy of cisplatin in in vitro and in vivo ovarian cancer models. J. Biol. Chem. 279, 23477–23485 (2004).
Kikuchi, E. et al. Suppression of hormone-refractory prostate cancer by a novel nuclear factor κB inhibitor in nude mice. Cancer Res. 63, 107–110 (2003).
Arlt, A. et al. Role of NF-κB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene 22, 3243–3251 (2003).
Wang, W., Cassidy, J. O'Brien, V., Ryan, K. M. & Collie-Duguid, E. Mechanistic and predictive profiling of 5-fluorouracil resistance in human cancer cells. Cancer Res. 64, 8167–8176 (2004).
Rayet, B. & Gelinas, C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene 18, 6938–6947 (1999).
Webster, G. & Perkins, N. Transcriptional cross-talk between NF-κB and p53. Mol. Cell. Biol. 19, 3485–3495 (1999).
Tergaonkar, V., Pando, M., Vafa, O., Wahl, G. & Verma, I. p53 stabilization is decreased upon NFκB activation: a role for NFκB in acquisition of resistance to chemotherapy. Cancer Cell 1, 493–503 (2002).
Karin, M., Cao, Y., Greten, F. & Li, Z. NF-κB in cancer: from innocent bystander to major culprit. Nature Rev. Cancer 2, 301–310 (2002).
Deveraux, Q., Reed, J. IAP family proteins-suppressors of apoptosis. Gene. Dev. 13, 239–252 (1999).
Cheng, Q., Lee, H., Li, Y., Parks, T. & Cheng, G. Upregulation of Bcl-xl and Bfl-1 as a potential mechanism of chemoresistance, which can be overcome by NF-κB inhibition. Oncogene 19, 4936–4940 (2000).
Cusack, J. C., Liu, R., & Baldwin, A. S. Inducible chemoresistance to 7-ethyl-10-(4-(1-piperidino)-1-piperidino)-carbonyloxycamptothecin (CPT-11) in colorectal cancer cells and a xenograft model is overcome by inhibition of nuclear factor-κB activation. Cancer Res. 60, 2323–2330 (2000).
Donnellan, R. & Chetty, R. Cyclin D1 and human neoplasia. Mol. Pathol. 51, 1–7 (1998).
Izzo, J. G. et. al. Dysregulated cyclin D1 expression early in head and neck tumorigenesis: in vivo evidence for an association with subsequent gene amplification. Oncogene 17, 2313–2322 (1998).
Takeshita, H. et al. Matrixmetallopoteinase 9 expression is induced by Epstein-Barr virus latent membrane protein 1 C-terminal activation regions 1 and 2. J. Viol. 73, 5548–5555 (1999).
Rangaswami, H. Bulbule, A. & Kundu, G. C. Nuclear factor-inducing kinase plays a crucial role in osteopontin-induced MAPK/IκBα kinase-dependent nuclear factor κB-mediated promatrix metalloproteinases-9 activation. J. Biol. Chem. 279, 38921–38935 (2004).
Keely, P., Westwick, J., Whitehead, I., Der, C. & Parise, L. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature 390, 632–636 (1997).
Huang, S., DeGuzman, A., Bucana, C. & Fidler, I. NF-κB activity correlates with growth, angiogenesis, and metastasis of human melanoma cells in nude mice. Clin. Cancer Res. 6, 2573–2581 (2000).
Bentires-Alj, M. et al. NF-κB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene 22, 90–97 (2000).
Wang, C. Y., Cusack, J. C., Liu, R. & Baldwin, A. S. Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NFκB. Nature Med. 5, 412–417 (1999).
Guo, J., Verma, U. N. Gaynor, R. B., Frenkel, E. P. & Becerra, C. R. Enhanced chemosensitivity to irinotecan by RNA interference mediated downregulation of the NF-κB p65 subunit. Clin. Cancer Res. 10, 3333–3341 (2004).
Cusack, J. C. et al. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-κB inhibition. Cancer Res. 61, 3535–3540 (2001). Shows that the proteasome inhibitor PS-341 (bortezomib) leads to the augmentation of chemosensitivity to irinotecan in vivo colon cancer xenografts mouse model.
Shah, S. A. et al. 26S proteasome inhibition induces apoptosis and limits growth of human pancreatic cancer. J. Cell Biochem. 82, 110–122 (2001).
Bold, R. J., Virudachalam, S. & McConkey, D. J. Chemosensitization of pancreatic cancer by inhibition of the 26S proteasome. J. Surg. Res. 100, 11–17 (2001).
Karin, M. & Ben-Neriah, Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Ann. Rev. Immunol. 18, 621–663 (2000).
Adams, J. et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 59, 2615–2622 (1999).
Zheng, B. et al. Induction of cell cycle arrest and apoptosis by the proteasome inhibitor PS-341 in Hodgkin disease cell lines is independent of Inhibitor of nuclear factor-κB mutations or activation of the CD30, CD40, and RANK receptors. Clin. Cancer Res. 10, 3207–3215 (2004).
Adams, J. The proteasome: a suitable antineoplastic target? Nature Rev. Cancer 4, 349–360 (2004).
Hideshima, T. et al. NF-κB as a therapeutic target in multiple myeloma. J. Biol. Chem. 277, 16639–16647 (2002).
Orlowski, R. Z. et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J. Clin. Oncol. 20, 4420–4427 (2002).
Richardson, P. G. et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N. Engl. J. Med. 348, 2609–2617 (2003).
Denlinger, C. E., Keller, M. D., Mayo, M. W., Broad, R. M. & Jones, D. R. Combined proteasome and histone deacetylase inhibition in non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 127, 1078–1086 (2004).
Denlinger, C. E., Rundall, B. K., Keller, M. D. & Jones, D. R. Proteasome inhibition sensitises non-small cell lung cancer to gemcitabine-induced apoptosis. Ann. Thorac. Surg. 78, 1207–1214 (2004).
Guzman, M. L. et al. Preferential induction of apoptosis for primary human leukemic stem cells. Proc. Natl Acad. Sci. USA 99, 16220–16225 (2002).
Lin, Z. P. et al. Prevention of brefeldin A-induced resistance to teniposide by the proteasome inhibitor MG-132: involvement of NF-κB activation in drug resistance. Cancer Res. 58, 3059–3065 (1998).
Pierce, J. W. et al. Novel inhibitors of cytokine-induced IκBα phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J. Biol. Chem. 272, 21096–21103 (1997).
Scaife, C. L. et al. Nuclear factor κB inhibitors induce adhesion-dependent colon cancer apoptosis: implications for metastasis. Cancer Res. 62, 6870–6878 (2002).
Lowe, S. W., Cepero, E & Evan G. Intrinsic tumour suppression. Nature 432, 307–315 (2004).
Mori, N. et al. Constitutive activation of NF-κB in primary adult T-cell leukaemia cells. Blood 93, 2360–2368 (1999).
Mori, N. et al. Bay 11-7082 inhibits transcription factor NF-κB and induces apoptosis of HTLV-I-infected T-cell lines and primary adult T-cell leukemia cells. Blood 100, 1828–1834 (2002).
Rundall, B. K., Denlinger, C. E. & Jones, D. R. Combined histone deacetylase and NF-κB inhibition sensitizes non-small cell lung cancer to cell death. Surgery 136, 416–425 (2004).
Pierce, J. W. et al. Novel inhibitors of cytokine-induced IκBα phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J. Biol. Chem. 272, 21096–21103 (1997).
Dai, Y. et al. Interruption of the NF-κB pathway by Bay 11-7082 promotes UCN-01-mediated mitochondrial dysfunction and apoptosis in human multiple myeloma cells. Blood 103, 2761–2770 (2004).
Nguyen, D. M. et al. Potentiation of paclitaxel cytotoxicity in lung and esophageal cancer cells by pharmacologic inhibition of the phosphoinositide 3-kinase/protein kinase B (Akt)-mediated signaling pathway. J. Thorac. Cardiovasc. Surg. 127, 365–375 (2004).
Scott, K. A. et al. An anti-tumor necrosis factor-α antibody inhibits the development of experimental skin tumors. Mol. Cancer Ther. 2, 445–451 (2003).
Szlosarek, P. & Balkwill, F. Tumour necrosis factor α: a potential target for the therapy of solid tumours. Lancet Oncol. 4, 565–573 (2003).
Brown, K., Gerstberger, S., Carlson, L., Franzoso, G. & Siebenlist, U. Control of IκB-1α proteolysis by site-specific, signal-induced phosphorylation. Science 267, 1485–1488 (1995).
Oya, M. et al. Constitutive activation of nuclear factor-κB prevents TRAIL-induced apoptosis in renal cancer cells. Oncogene 20, 3888–3896 (2001).
Auphan, N., DiDonato, J. A., Rosette, C., Helmberg, A. & Karin, M. Immunosuppression by glucocorticoids: inhibition of NFκB activity through inhibition of IκB synthesis. Science 270, 286–290 (1995).
Gasparini, G., Longo, R., Sarmiento, R. & Morabito, A. Inhibitors of cyclo-oxygenase 2: a new class of anticancer agents? Lancet Oncol. 4, 605–615 (2003).
Yin, M. J., Yamamoto, Y. & Gaynor, R. B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature 396, 77–80 (1998).
Mitchell, J. A., Akarasereenont, P., Thiermermann, C., Flower, R. J. & Vane, J. R. Selectivity of nonsteroidal anti-inflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc. Natl Acad. Sci. USA 90, 11693–11697 (1994).
Kalgutkar, A. S. et al. Aspirin-like molecule that covalently inactivates cyclooxygenase-2. Science 280, 1268–1270 (1998).
Yamamoto, Y., Yin, M. -J., Lin, K. M. & Gaynor, R. B. Sulindac inhibits activation of the NF-κB pathway. J. Biol. Chem. 274, 27307–27314 (1999).
Shishodia, S., Koul, D. & Aggarwal, B. B. Cyclooxygenase (COX)-2 inhibitor celecoxib abrogates TNF-induced NF-κB activation through inhibition of activation of IκBα kinase and Akt in human non-small cell lung carcinoma: correlation with suppression of COX-2 synthesis. J. Immunol. 173, 2011–2022 (2004). Shows that the COX2 inhibitor celecoxib suppresses NF-κB activation induced by a wide range of agents in various cell types, leading to downregulation in the synthesis of COX2.
Wahl, C., Liptay, S., Adler, G. & Schmid, R. M. Sulufasalazine: a potent and specific inhibitor of nuclear factor κB. J. Clin. Invest. 101, 1163–1174 (1998).
Weber, C. K., Liptay, S., Wirth, T., Adler, G. & Schmid, R. M. Suppression of NF-κB activity by sulfasalazine is mediated by direct inhibition of IκB kinases α and β. Gastroenterology 119, 1209–1218 (2000).
Hovstadius, P. et al. A phase I study of CHS 828 in patients with solid tumor malignancy. Clin. Cancer Res. 8, 2843–2850 (2002).
Olsen, L. S. et al. Anticancer agent CHS 828 suppresses nuclear factor-κB activity in cancer cells through downregulation of IKK activity. Int. J. Cancer 111, 198–205 (2004).
Mrtinsson, P., Ekelund, S., Nygren, P. & Larsson, R. The combination of the antitumoural pyridyl cyanoguanidine CHS828 and etoposide in vitro-from cytotoxic synergy to complete inhibition of apoptosis. Br. J. Pharmacol. 137, 568–573 (2002).
Decker, R. H., Dai, Y. & Grant, S. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in human leukemia cells (U937) through the mitochondrial rather than the receptor-mediated pathway. Cell Death Differ. 8, 715–724 (2001).
Thomas, J. P. et al. Phase I clinical and pharmacokinetic trial of the cyclin-dependent kinase inhibitor flavopiridol. Cancer Chemother. Pharmacol. 50, 465–472 (2002).
Karp, J. E. et al. Timed sequential therapy of acute leukemia with flavopiridol: in vitro model for a phase I clinical trial. Clin. Cancer Res. 9, 307–315 (2003).
Takada, Y. & Aggarwal, B. B. Flavopiridol inhibitis NF-κB activation induced by various carcinogens and inflammatory agents through inhibition of IκBα kinase and p65 phosphorylation: abrogation of cyclin D1, cyclooxygenase-2, and matrix metalloprotease-9. J. Biol. Chem. 279, 4750–4759 (2004).
Gao, N., Dai, Y., Rahmani, M., Dent, P. & Grant S. Contribution of disruption of the nuclear factor-κB pathway to induction of apoptosis in human leukemia cells by histone deacetylase inhibitors and flavopiridol. Mol. Pharmacol. 66, 956–963 (2004).
Motwani, M., Delohery, T. M. & Schwartz, G. K. Sequential dependent enhancement of caspase activation and apoptosis by flavopiridol on paclitaxel-treated human gastric and breast cancer cells. Clin. Cancer Res. 5, 1876–1883 (1999).
Rosato, R. R. et al. The cyclin-dependent kinase inhibitor flavopiridol disrupts sodium butyrate-induced p21WAF1/CIP1 expression and maturation while reciprocally potentiating apoptosis in human leukemia cells. Mol. Cancer Ther. 1, 253–266 (2002).
Erkel, G., Anke, T. & Sterner, O. Inhibition of NFκB activation by panepoxydone. Biochem. Biophys. Res. Comm. 226, 214–221 (1996).
Matsumoto, N. et al. Synthesis of NFκB activation inhibitors derived from epoxyquinomicin C. Bioog. Med. Chem. Lett. 10, 865–869 (2000).
Ariga, A., Namekawa, J., Matsumoto, N., Inoue, J. & Umezawa, K. Inhibition of tumor necrosis factor-α-induced nuclear translocation and activation of NFκB by dehydroxymethylepoxyquinomicin. J. Biol. Chem. 277, 24625–24630 (2002).
Pati, S. et al. Antitumorigenic effects of HIV protease inhibitor ritonavir: inhibition of Kaposi sarcoma. Blood 99, 3771–3779 (2002).
Sgadari, C. et al. HIV protease inhibitors are potent antiangiogenic molecules and promote regression of Kaposi sarcoma. Nature Med. 8, 225–232 (2002).
Pajonk, F., Himmelsbach, J., Riess, K., Sommer, A. & McBride W. H. the human immunodeficiendy virus (HIV)-1 protease inhibitor saquinavir inhibits proteasome function and cause apoptosis and radiosensitization in non-HIV-associated human cancer cells. Cancer Res. 62, 5230–5235 (2002).
Ikezoe, T. et al. HIV-1protease inhibitor, ritonavir: a potent inhibitor of CYP3A4, enhanced the anticancer effects of docetaxel in androgen-independent prostate cancer cells in vitro and in vivo. Cancer Res. 64, 7426–7431 (2004).
Kumar, G. N., Rodrigues, A. D., Buko, A. M. & Denissen, J. F. Cytochrome P450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsome. J. Pharmaco. Exp. Ther 277, 423–431 (1996).
Li, Y. et al. Apoptosis-inducing effect of chemotherapeutic agents is potentiated by soy isoflavone genistein, a natural inhibitor of NF-κB in BxPC-3 pancreatic cancer cell line. Pancreas 28, e90–95 (2004).
Sigala, J. L. D. et al. Activation of transcription factor NF-κB requires ELKS, an IκB kinase regulatory subunit. Science 304, 1963–1967 (2004).
Romano, M. F. et al. Rapamycin inhibits doxorubicin-induced NF-κB-Rel nuclear activity and enhances the apoptosis of melanoma cells. Eur. J. Cancer 40, 2829–2836 (2004).
Barlogie, B. et al. Treatment of multiple myeloma. Blood 103, 20–32 (2004).
Palanki, M. S. et al. structure-activity relationship studies of ethyl 2-[(3-methyl-2,5-dioxo(3-pyrrolinyl))amino]-4-(trifluoromethyl)pyrimidine-5-carboxylate: an inhibitor of AP-1 and NF-κB mediated gene expression. Bioorg. Med. Chem. Lett. 12, 2573–2577 (2002).
Castro, A. C. et al. Novel IKK inhibitors: β-carbolines. Boorg. Med. Chem. Lett. 13, 2491–2422 (2003).
Burke, J. R. et al. BMS-345541 is a highly selective inhibitor of IκB kinase that binds at an allosteric site of the enzyme and blocks NF-κB-dependent transcription in mice. J. Biol. Chem. 278, 1450–1456 (2003).
Kishore, N. et al. A selective IKK-2 inhibitor blocks NF-κB-dependent gene expression in interleukin-1β-stimulated synovial fibroblasts. J. Biol. Chem. 278, 32861–32871 (2003).
Shigeno, M. et al. Interferon-α sensitises human hepatoma cells to TRAIL-induced apoptosis through DR5 upregulation and NF-κB inactivation. Oncogene 22, 1653–1662 (2003).
Lentsch, A. B., Shanley, T. P., Sarma, V. & Ward, P. A. In vivo suppression of NF-κB and presentation of I-κBα by interleukin-10 and interleukin-13. J. Clin. Invest. 100, 2443–2448 (1997).
Manna, S. K & Aggarwal, B. B. IL-13 suppresses TNF-induced activation of nuclear factor-κB, activation protein-1, and apoptosis. J. Immunol. 161, 2863–2872 (1998).
Molina-Holgado, E. et al. Interleukin-4 and interleukin-10 modulate nuclear factor κB activity and nitric oxide synthase-2 expression in Theiler's virus-infected brain astrocytes. J. Neurochem. 81, 1242–1252 (2002).
Teare, K. A., Pearson, R. G., Shakesheff, K. M. & Haycock, J. W. α-MSH inhibits inflammatory signalling in schwann cells. Neuroreport. 15, 493–498 (2004).
Rao, Ch. V., Li, X. Manna, S. K., Lei, Z. M. & Aggarwal, B. B. Human chorionic gonadotropin decreases proliferation and invasion of breast cancer MCF-7 cells by inhibiting NF-κB and AP-1 activation. J. Biol. Chem. 279, 25503–25510 (2004).
Haeffner, A. et al. Inhibitory effect of growth hormone on TNF-α secretion and nuclear factor-κB transcription in lipopolysaccharide-stimulated human monocytes. J. Immunol. 158, 1310–1314 (1997).
Cherbonier, C. et al. Potentiation of tumour apoptosis by human growth hormone via glutathione production and decreased NF-κB activity. Br. J. Cancer 89, 1108–1115 (2003).
Li, W. G. et al. Ghrelin inhibits Proinflammatory responses and nuclear factor-κB activation in human endothelial cells. Circulation 109, 2221–2226 (2004).
De Smaele, E. et al. Induction of gadd 45β by NF-κB downregulates pro-apoptotic JNK signalling. Nature 414, 308–313 (2001).
Tang, G. et al. Inhibition of JNK activation through NFκB target genes. Nature 414, 313–317 (2001).
Sanchez-Perez, I., Benitah, S. A., Martinez-Gomariz, M., Lacal, J. C. & Perona, R. Cell stress and MEKK1-mediated c-Jun activation modulate NFκB activity and cell viability. Mol. Biol. Cell. 13, 2933–2945 (2002).
Tergaonkar, V., Bottero, V., Ikawa, M., Li, Q. & Verma, I. M. IκB kinase-independent IκBα degradation pathway: functional NF-κB activity and implications for cancer therapy. Mol. Cell. Biol. 23, 8070–8083 (2003). Indicates that NF-κB activation by chemotherapeutic agents is mediated by IKK-dependent and -independent pathways.
Brach, M. A. et al. Ionizing radiation induces expression and binding activity of the nuclear factor κB. J. Clin. Invest. 88, 691–695 (1991).
Kerbel, R. S. & Kamen, B. A. The anti-angiogenic basis of metronomic chemotherapy. Nature Rev. Cancer 4, 423–436 (2004).
Campbell, K. J., Rocha, S. & Perkins, N. D. Active repression of antiapoptotic gene expression by RelAp65 NFR-κB. Mol. Cell 13, 853–865 (2004).
Turco, M. C. et al. NF-κB/Rel-mediated regulation of apoptosis in hematologic malignancies and normal hematopoietic progenitors. Leukemia 18, 11–17 (2004).
Das, K. C. & White, C. W. Activation of NF-κB by antineoplastic agents. J. Biol. Chem. 272, 14914–14920 (1997).
Piret, B. & Piette, J. Topoisomerase poisons activate the transcription factor NF-κB in ACH-2 and CEM cells. Nucleic Acids Res. 24, 4242–4248 (1996).
Wang, C. Y., Mayo, M. W. & Baldwin, A. S. Jr. TNF-α and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science 274, 784–787 (1996).
Rosette, C. & Karin, M. Cytoskeletal control of gene expression: depolymerization of microtubules activates NF-κB. J. Cell Biol. 128, 1111–1119 (1995).
Fahy, B. N., Schkieman, M. G., Virudachalam, S. & Bold, R. J. Inhibition of Akt abrogates chemotherapy-induced NF-κB survival mechanism: implications for therapy in pancreatic cancer. J. Am. Coll. Surg. 198, 591–599 (2004).
Mayo, M. W. et al. Ineffectiveness of hitsone deacetylase inhibitors to induce apoptosis involves the transcriptional activation of NF-κB through the Akt pathway. J. Biol. Chem. 278, 18980–18989 (2003).
Adam, E. et al. Potentiation of tumor necrosis factor-induced NF-κB activation by deacetylase inhibitors is associated with a delayed cytoplasmic reappearance of IκBα. Mol. Cell. Biol. 23, 6200–6209 (2003).
Gong, L., Li, Y., Kurepa, A. N. & Sarkar, F. H. Inactivation of NF-κB by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene 22, 4702–4709 (2003).
Watabe, M., Hishikawa, K., Takayanagi, A., Shimizu, N. & Nakaki, T. Caffeic acid phenethyl ester induces apoptosis by inhibition of NFκB and activation of Fas in human breast cancer MCF-7 cells. J. Biol. Chem. 279, 6017–6026 (2004).
Natarajan, K., Singh, S., Burke, T. R. Jr., Grunberger, D. & Aggarwal, B. B. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Proc. Natl Acad. Sci. USA 93, 9090–9095 (1996).
Srivastava, S. K. & Singh, S. V. Cell cycle arrest, apoptosis induction and inhibition of nuclear factor κB activation in anti-proliferative activity of benzyl isothiocyanate against human pancreatic cancer cells. Carcinogenesis 25, 1701–1709 (2004).
Holmes-McNary, M. & Baldwin, A. S. Jr. Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IκB kinase. Cancer Res. 60, 3477–3483 (2000).
Estrov, Z. et al. Resveratrol blocks interleukin-1α-induced activation of the nuclear transcription factor NF-κB, inhibits proliferation, cause S-phase arrest, and induces apoptosis of acute myeloid leukaemia cells. Blood 102, 987–995 (2003).
Li, L., Aggarwal, B. B., Shishodia, S, Abbruzzese, J. & Kurzrock, R. Nuclear factor-κB and IκB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer 101, 2351–2362 (2004).
Aggarwal, S., Takada, Y., Singh, S., Myers, J. N. and Aggarwal, B. B. Inhibition of growth and survival of human head and neck squamous cell carcinoma cells by curcumin via modulation of nuclear factor-κB signalling. Int. J. Cancer 111, 679–692 (2004).
Saleem, M., Afaq, F., Adhami, V. M. & Mukhtar, H. Lupeol modulates NF-κB and PI3K/Akt pathways and inhibits skin cancer in CD-1 mice. Oncogene 23, 5203–5214 (2004).
Aktas, O. et al. Green tea epigallocatechin-3-gallate mediates T cellular NF-κB inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J. Immunol. 173, 5794–5800 (2004).
Kim, G. Y. et al. Lycopene suppresses the lipopolysaccharide-induced phenotypic and functional maturation of murine dendritic cells through inhibition of mitogen-activated protein kinases and nuclear factor-κB. Immunology 113, 203–211 (2004).
Minekawa, R. et al. Human breast milk suppresses the transcriptional regulation of IL-1β-induced NF-κB signalling in human intestinal cell. Am. J. Physiol. Cell Physiol. 287, C1404–C1411 (2004).
Xia, Y. F. et al. Andrographolide attenuates inflammation by inhibition of NF-κB activation through covalent modification of reduced cystein 62 of p50. J. Immunol. 173, 4207–4217 (2004).
Shishodia, S. & Aggarwal, B. B. Guggulsterone inhibits NF-κB and IκB kinase activation, suppresses expression of anti-apoptotic gene products, and enhances apoptosis. J. Biol. Chem. 279, 47148–47158 (2004).
Siedle, B. et al. Quantitative structure-activity relationship of sesquiterpene lactones as inhibitors of the nuclear factor NF-κB. J. Med. Chem. 47, 6042–6054 (2004).
Gehrt, A., Erkel, G., Anke, T. & Sterner, O. Cycloepoxydon, 1-hydroxy-2-hydroxymethyl-3-pent-1-enylbenzene and 1-hydroxy-2-hydroxymethyl-3-pent-1,3-dienylbenzene, new inhibitors of eukaryotic signal transduction. J. Antibiot. (Tokyo) 51, 455–463 (1998).
Pahl, H. L. et al. The immunosuppressive fungal metabolite gliotoxin specifically inhibits transcription factor Nk-κB. J. Exp. Med. 183, 1829–1840 (1996).
Cheng, A. L. et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 21, 2895–2900 (2001).
Acknowledgements
We thank N. Aoki and N. Kanamoto for kind help. This work was supported by a grant for optimization of new anticancer drugs from the Health and Labour Sciences Research Grants of Third Term Comprehensive Control Research for Cancer from the Ministry of Health, Labour and Welfare.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- ANKYRIN REPEAT
-
A repeating sequence of 30–33 amino acids that is found in the ankyrin protein. The ankyrin repeat of IκB proteins is required for association with the nuclear localization signal of NF-κB proteins.
- PROTEASOME
-
A 26S multiprotein complex that catalyses the breakdown of polyubiquitylated proteins.
- COLITIS-ASSOCIATED CANCER
-
Colitis-associated cancer arises in patients with inflammatory bowel disease, particularly ulcerative colitis. The risk of colorectal cancer in patients with ulcerative colitis 20 years after the initial diagnosis is estimated to be between 5% and 10%.
- CYCLOOXYGENASE-2
-
Cyclooxygenase-2 (COX2) is the enzyme that normally synthesizes prostaglandins during an inflammatory response. Proinflammatory and mitogenic stimuli, such as growth factors and cytokines, induce COX2 and many tumours express COX2.
- UBIQUITIN
-
A 76-amino-acid polypeptide that is highly conserved in eukaryotic cells. The ubiquitin system is a conserved complex that marks old or defective proteins for cleavage.
- OFF-TARGET EFFECT
-
This term is used to describe the effect of a drug on another protein, molecule or complex that is different from the protein that it is known to effect or originally designed to effect.
- PYRIDYL CYANOGUANIDINE
-
Pyridyl cyanoguanidines are potassium-channel openers. Pinacidil (N-1,2,2-trimethylpropyl-N-cyano-N′-4-pyridylguanidine) is a structural prototype with potent antihypertensive activity. Replacement of the side chain of pinacidil by longer aryl-containing side chains give rise to compounds with increasing antitumour activity but without antihypertensive activity.
- ELKS
-
ELKS is a 105-kDa IKK regulatory subunit protein. The name ELKS is derived from the relative abundance of its constitutive amino acids: glutamic acid (E), leucine (L), lysine (K) and serine (S). The function of ELKS is probably to recruit IκBα to the IKK complex.
- MACROLIDES
-
A group of antibiotics produced by various strains of Streptomyces that have a complex macrocyclic structure. They inhibit protein synthesis by blocking the 50S ribosomal subunit and include erythromycin and carbomycin.
- IMMUNOPHILINS
-
Receptors for immunosuppressive drugs such as cyclosporin A and rapamycin. Drug– immunophilin complexes suppress the immune response.
Rights and permissions
About this article
Cite this article
Nakanishi, C., Toi, M. Nuclear factor-κB inhibitors as sensitizers to anticancer drugs. Nat Rev Cancer 5, 297–309 (2005). https://doi.org/10.1038/nrc1588
Issue Date:
DOI: https://doi.org/10.1038/nrc1588
This article is cited by
-
N6-methyladenosine of Spi2a attenuates inflammation and sepsis-associated myocardial dysfunction in mice
Nature Communications (2023)
-
Macrophages-aPKCɩ-CCL5 Feedback Loop Modulates the Progression and Chemoresistance in Cholangiocarcinoma
Journal of Experimental & Clinical Cancer Research (2022)
-
Gelidiella acerosa Compounds Target NFκB Cascade in Lung Adenocarcinoma
Applied Biochemistry and Biotechnology (2022)
-
FSTL1 increases cisplatin sensitivity in epithelial ovarian cancer cells by inhibition of NF-κB pathway
Cancer Chemotherapy and Pharmacology (2021)
-
Synthetic curcumin analog: inhibiting the invasion, angiogenesis, and metastasis in human laryngeal carcinoma cells via NF-kB pathway
Molecular Biology Reports (2021)