Abstract
The cAMP and cAMP-dependent protein kinase A (PKA) signaling cascade is a ubiquitous pathway acting downstream of multiple neuromodulators. We found that the phosphorylation of phosphodiesterase-4 (PDE4) by cyclin-dependent protein kinase 5 (Cdk5) facilitated cAMP degradation and homeostasis of cAMP/PKA signaling. In mice, loss of Cdk5 throughout the forebrain elevated cAMP levels and increased PKA activity in striatal neurons, and altered behavioral responses to acute or chronic stressors. Ventral striatum– or D1 dopamine receptor–specific conditional knockout of Cdk5, or ventral striatum infusion of a small interfering peptide that selectively targeted the regulation of PDE4 by Cdk5, produced analogous effects on stress-induced behavioral responses. Together, our results demonstrate that altering cAMP signaling in medium spiny neurons of the ventral striatum can effectively modulate stress-induced behavioral states. We propose that targeting the Cdk5 regulation of PDE4 could be a new therapeutic approach for clinical conditions associated with stress, such as depression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Conti, M. & Beavo, J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu. Rev. Biochem. 76, 481–511 (2007).
Houslay, M.D. Underpinning compartmentalized cAMP signaling through targeted cAMP breakdown. Trends Biochem. Sci. 35, 91–100 (2010).
Sette, C. & Conti, M. Phosphorylation and activation of a cAMP-specific phosphodiesterase by the cAMP-dependent protein kinase. Involvement of serine 54 in the enzyme activation. J. Biol. Chem. 271, 16526–16534 (1996).
MacKenzie, S.J. et al. Long PDE4 cAMP-specific phosphodiesterases are activated by protein kinase A–mediated phosphorylation of a single serine residue in upstream conserved region 1 (UCR1). Br. J. Pharmacol. 136, 421–433 (2002).
Bibb, J.A. et al. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature 402, 669–671 (1999).
Dhavan, R. & Tsai, L.H. A decade of CDK5. Nat. Rev. Mol. Cell Biol. 2, 749–759 (2001).
Angelo, M., Plattner, F. & Giese, K.P. Cyclin-dependent kinase 5 in synaptic plasticity, learning and memory. J. Neurochem. 99, 353–370 (2006).
Plattner, F. et al. Memory enhancement by targeting Cdk5 regulation of NR2B. Neuron 81, 1070–1083 (2014).
Meyer, D.A. et al. Ischemic stroke injury is mediated by aberrant Cdk5. J. Neurosci. 34, 8259–8267 (2014).
Barnett, D.G. & Bibb, J.A. The role of Cdk5 in cognition and neuropsychiatric and neurological pathology. Brain Res. Bull. 85, 9–13 (2011).
Su, S.C. & Tsai, L.H. Cyclin-dependent kinases in brain development and disease. Annu. Rev. Cell Dev. Biol. 27, 465–491 (2011).
Fischer, A., Sananbenesi, F., Schrick, C., Spiess, J. & Radulovic, J. Cyclin-dependent kinase 5 is required for associative learning. J. Neurosci. 22, 3700–3707 (2002).
Bignante, E.A. et al. Involvement of septal Cdk5 in the emergence of excessive anxiety induced by stress. Eur. Neuropsychopharmacol. 18, 578–588 (2008).
Bignante, E.A., Paglini, G. & Molina, V.A. Previous stress exposure enhances both anxiety-like behavior and p35 levels in the basolateral amygdala complex: modulation by midazolam. Eur. Neuropsychopharmacol. 20, 388–397 (2010).
Zhu, W.L. et al. Increased Cdk5/p35 activity in the dentate gyrus mediates depressive-like behavior in rats. Int. J. Neuropsychopharmacol. 15, 795–809 (2012).
Zhong, P. et al. Cyclin-dependent kinase 5 in the ventral tegmental area regulates depression-related behaviors. J. Neurosci. 34, 6352–6366 (2014).
Carlson, J.N., Fitzgerald, L.W., Keller, R.W. Jr. & Glick, S.D. Side and region dependent changes in dopamine activation with various durations of restraint stress. Brain Res. 550, 313–318 (1991).
Chrapusta, S.J., Wyatt, R.J. & Masserano, J.M. Effects of single and repeated footshock on dopamine release and metabolism in the brains of Fischer rats. J. Neurochem. 68, 2024–2031 (1997).
Tye, K.M. et al. Dopamine neurons modulate neural encoding and expression of depression-related behavior. Nature 493, 537–541 (2013).
Chaudhury, D. et al. Rapid regulation of depression-related behaviors by control of midbrain dopamine neurons. Nature 493, 532–536 (2013).
Hawasli, A.H. et al. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat. Neurosci. 10, 880–886 (2007).
Weber, P., Metzger, D. & Chambon, P. Temporally controlled targeted somatic mutagenesis in the mouse brain. Eur. J. Neurosci. 14, 1777–1783 (2001).
Nestler, E.J. & Hyman, S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 13, 1161–1169 (2010).
Schlaepfer, T.E. et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 33, 368–377 (2008).
Nestler, E.J. & Carlezon, W.A. Jr. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 59, 1151–1159 (2006).
Mungenast, A.E. & Tsai, L.H. Cognitive function in health and disease: the role of epigenetic mechanisms. Neurodegener. Dis. 10, 191–194 (2012).
Song, G.G., Kim, J.H. & Lee, Y.H. Genome-wide pathway analysis in major depressive disorder. J. Mol. Neurosci. 51, 428–436 (2013).
Hek, K. et al. A genome-wide association study of depressive symptoms. Biol. Psychiatry 73, 667–678 (2013).
Dwivedi, Y. & Pandey, G.N. Adenylyl cyclase-cyclicAMP signaling in mood disorders: role of the crucial phosphorylating enzyme protein kinase A. Neuropsychiatr. Dis. Treat. 4, 161–176 (2008).
Duman, R.S. & Voleti, B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci. 35, 47–56 (2012).
Fujita, M. et al. Downregulation of brain phosphodiesterase type IV measured with (11)C-(R)-rolipram positron emission tomography in major depressive disorder. Biol. Psychiatry 72, 548–554 (2012).
O'Donnell, J.M. & Xu, Y. Evidence for global reduction in brain cyclic adenosine monophosphate signaling in depression. Biol. Psychiatry 72, 524–525 (2012).
Halene, T.B. & Siegel, S.J. PDE inhibitors in psychiatry–future options for dementia, depression and schizophrenia? Drug Discov. Today 12, 870–878 (2007).
Kleppisch, T. Phosphodiesterases in the central nervous system. Handb. Exp. Pharmacol. 191, 71–92 (2009).
Zeller, E., Stief, H.J. & Pflug, B. Sastre-y-Hernandez, M. Results of a phase II study of the antidepressant effect of rolipram. Pharmacopsychiatry 17, 188–190 (1984).
Zhang, H.T. et al. Antidepressant-like profile and reduced sensitivity to rolipram in mice deficient in the PDE4D phosphodiesterase enzyme. Neuropsychopharmacology 27, 587–595 (2002).
O'Donnell, J.M. & Zhang, H.T. Antidepressant effects of inhibitors of cAMP phosphodiesterase (PDE4). Trends Pharmacol. Sci. 25, 158–163 (2004).
Houslay, M.D., Schafer, P. & Zhang, K.Y. Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov. Today 10, 1503–1519 (2005).
Burgin, A.B. et al. Design of phosphodiesterase 4D (PDE4D) allosteric modulators for enhancing cognition with improved safety. Nat. Biotechnol. 28, 63–70 (2010).
Nestler, E.J. et al. Neurobiology of depression. Neuron 34, 13–25 (2002).
Mayberg, H.S. et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am. J. Psychiatry 156, 675–682 (1999).
Schmidt, E.F. et al. Identification of the cortical neurons that mediate antidepressant responses. Cell 149, 1152–1163 (2012).
Warner-Schmidt, J.L. et al. Cholinergic interneurons in the nucleus accumbens regulate depression-like behavior. Proc. Natl. Acad. Sci. USA 109, 11360–11365 (2012).
Bibb, J.A. et al. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature 410, 376–380 (2001).
Benavides, D.R. et al. Cdk5 modulates cocaine reward, motivation, and striatal neuron excitability. J. Neurosci. 27, 12967–12976 (2007).
Kim, S.H. & Ryan, T.A. CDK5 serves as a major control point in neurotransmitter release. Neuron 67, 797–809 (2010).
Tan, T.C. et al. Cdk5 is essential for synaptic vesicle endocytosis. Nat. Cell Biol. 5, 701–710 (2003).
Johansson, E.M., Reyes-Irisarri, E. & Mengod, G. Comparison of cAMP-specific phosphodiesterase mRNAs distribution in mouse and rat brain. Neurosci. Lett. 525, 1–6 (2012).
Mathews, D.C., Henter, I.D. & Zarate, C.A. Targeting the glutamatergic system to treat major depressive disorder: rationale and progress to date. Drugs 72, 1313–1333 (2012).
Castagne, V., Moser, P., Roux, S. & Porsolt, R.D. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr. Protoc. Neurosci. 8.8, 10A (2011).
Golden, S.A., Covington, H.E. III., Berton, O. & Russo, S.J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 6, 1183–1191 (2011).
Willner, P., Towell, A., Sampson, D., Sophokleous, S. & Muscat, R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl.) 93, 358–364 (1987).
Yuen, E.Y. et al. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 73, 962–977 (2012).
Nishi, A., Snyder, G.L. & Greengard, P. Bidirectional regulation of DARPP-32 phosphorylation by dopamine. J. Neurosci. 17, 8147–8155 (1997).
Nishi, A. et al. Distinct roles of PDE4 and PDE10A in the regulation of cAMP/PKA signaling in the striatum. J. Neurosci. 28, 10460–10471 (2008).
Frank-Cannon, T.C. et al. Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J. Neurosci. 28, 10825–10834 (2008).
Marchmont, R.J. & Houslay, M.D. A peripheral and an intrinsic enzyme constitute the cyclic AMP phosphodiesterase activity of rat liver plasma membranes. Biochem. J. 187, 381–392 (1980).
Lobban, M., Shakur, Y., Beattie, J. & Houslay, M.D. Identification of two splice variant forms of type-IVB cyclic AMP phosphodiesterase, DPD (rPDE-IVB1) and PDE-4 (rPDE-IVB2) in brain: selective localization in membrane and cytosolic compartments and differential expression in various brain regions. Biochem. J. 304, 399–406 (1994).
Jiang, L.I. et al. Use of a cAMP BRET sensor to characterize a novel regulation of cAMP by the sphingosine 1-phosphate/G13 pathway. J. Biol. Chem. 282, 10576–10584 (2007).
Murdoch, H. et al. Isoform-selective susceptibility of DISC1/phosphodiesterase-4 complexes to dissociation by elevated intracellular cAMP levels. J. Neurosci. 27, 9513–9524 (2007).
Zhao, Y., Zhang, W. & White, M.A. Capillary high-performance liquid chromatography/mass spectrometric analysis of proteins from affinity-purified plasma membrane. Anal. Chem. 75, 3751–3757 (2003).
Czernik, A.J., Mathers, J. & Mische, S.M. Phosphorylation state–specific antibodies. in Regulatory Protein Modification: Techniques and Protocols Neuromethods, Vol. 30 (ed. H.C. Hemmings, Jr.) 219–250 (Humana Press, 1997).
Acknowledgements
We thank N. Heintz (Rockefeller University) and GenSat for D1R-Cre mice, K. Deisseroth (Stanford University) for the Dio-Cre vector, C. Burger (University of Wisconsin-Madison) for AAV vectors, H. Ball and Y. Li (University of Texas Southwestern (UTSW) Protein Technology Center) for peptides, the UTSW Animal Resource Center for help with phosphorylation state–specific antibody generation, D.M. Dietz, M. Lutter, M. Kouser and J. Kumar for help with the social defeat procedure, and T. Singh and G. Mettlach for technical assistance. We thank M. Trivedi and the UTSW Depression Center for support. This work was supported by a Brain and Behavior Research Foundation NARSAD Young Investigator Award (K.H.), a pre-doctoral National Research Service Award from the National Institute on Drug Abuse (D.R.B.), a grant from the Darrell K Royal Research Fund for Alzheimer's Research (F.P.) and the California Metabolic Research Foundation (M.D.H.), as well as by US National Institutes of Health grants to A.C.N. and P.G. (MH090963, DA10044), E.J.N. (MH51399), R.T. (GM084249) and J.A.B. (MH79710, MH083711, DA016672, DA018343, DA033485, NS073855).
Author information
Authors and Affiliations
Contributions
F.P., K.H., D.R.B., A.H., T.C.T., C.T., J.D., M.W.F., E.Y.Y., M.S.G. and A.N. collected data and analyzed the experiments. F.P., Z.Y., A.C.N., E.J.N., A.N., P.G., R.T., M.D.H. and J.A.B. contributed to study design, supervision and interpretation of the experiments. F.P. and J.A.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
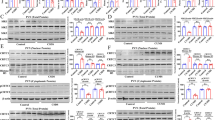
Supplementary Figure 1 PDE4 phosphorylation state-specific antibody generation, distribution of PDE4 in brain, and cGMP levels in striatal slices.
(a) Identification of the Cdk5-dependent phosphorylation site on PDE4B1 by LC-LC MS analysis. The presence of a single charged peptide (SDSDYDLpSPK) indicates the site of phosphorylation at amino acid residue Ser145 of the rat PDE4B1 sequence, with “pS” denoting the position of the phospho-serine. The spectrum depicts the y-ion series as a vertical line in the peptide sequence and corresponding site of y-ion generation (i.e. y1, y2, etc.). (b) Time-course of in vitro phosphorylation of recombinant PDE4B1 by PKA. Time-dependent 32P incorporation, Coomassie-stained (CBB) PDE4B1, and quantified reaction stoichiometry are shown. (c) PDE4 family member and Cdk5 brain tissue distribution. Immunoblots with pan-specific PDE4A, 4B, and 4D antibodies are shown with individual isoforms indicated. Blots for Cdk5 and GAPDH are also shown. ob, olfactory bulb; ctx, cortex; str, striatum; nac, nucleus accumbens; hip, hippocampus; cer, cerebellum. (d) Validation of phosphorylation state-specific antisera to phospho-Ser133 PDE4B1 [pPDE4 (PKA)] phosphorylated by PKA. Dot blot analysis to confirm phosphorylation state-specificity of antibody. Selective detection of phospho- versus dephospho-peptide with corresponding Pyronin Y staining of total peptide is shown. (e) Immunoblot of lysates from striatal slices incubated in the absence (Control) or presence of forskolin (10 µM, 10 min) probed with pPDE4 (PKA) antibody. (f) Validation of phosphorylation state-specific antisera to phospho-Ser145 PDE4B1 [pPDE4 (Cdk5)] phosphorylated by Cdk5. Immunoblot analysis of recombinant PDE4B1 phosphorylated (+) or mock phosphorylated (–) in vitro with Cdk5 using pPDE4 (Cdk5) versus total PDE4 antibodies (top panel). Blots of PC12 cells transfected with PDE4B1 treated with control buffer or Indo A (10 µM, 60 min) are shown in the bottom panel. (g) Cdk5 inhibition with indolinone A (IndoA, 10 µM) does not affect cGMP levels in the absence or presence of sodium nitroprusside (SNP), an activator of guanylate cyclases, in striatal slices (n = 4–8. All data shown are means ± s.e.m., *P < 0.05, **P < 0.01, ***P < 0.001.
Supplementary Figure 2 Reciprocal relationship of Cdk5 and PKA activity in striatum.
(a) Evaluation of PKA-mediated phosphorylation in response to treatment of striatal slices with 50 µM roscovitine. Representative blots for PKA-mediated phospho-Ser133 (pS133) CREB, phospho-Ser9 (pS9) synapsin, phospho-Thr34 (P-T34) DARPP-32, phospho-Ser845 (pS845) GluR1, and respective total protein are shown with quantification (n = 3–6). (b) The dose-dependent, reciprocal relationship between PKA- and Cdk5-dependent phosphorylation of DARPP-32. Immunoblots (left) of lysates from mouse striatal slices treated with the Cdk5 inhibitor roscovitine (0–50 µM, 60 min) for phospho-Thr75 (P-T75), phospho-Thr34 (P-T34), and total DARPP-32 (D-32) are shown with quantification (right). All data shown are means ± s.e.m., *P < 0.05, **P < 0.01, ***P < 0.001.
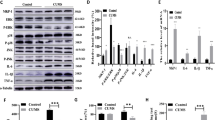
Supplementary Figure 3 Decreased Cdk5 activity and PDE4 phosphorylation in Cdk5 cKO.
(a) Immunoprecipitation Cdk5 kinase activity assay using striatal lysate from WT versus Cdk5 cKO. The effect of treatment of immunoprecipitate from control samples with Cdk5 inhibitor roscovitine (Ros, 10 μM) is also shown. Radiographic and Coomassie stained gels with quantification are shown (n = 6–9). (b, c) PDE4 phosphorylation levels at the Cdk5 (b) and PKA sites (c) in lysates of ventral striatum from Cdk5 cKO and control WT mice (n = 4). (d) Levels of cGMP are unaltered in striatum from Cdk5 cKO and control mice (n = 4-5). All data shown are means ± s.e.m., *P < 0.05, **P < 0.01, ***P < 0.001.
Supplementary Figure 4 PKA regulation of NMDA receptor currents is altered in Cdk5 cKO mice.
(a) Plot of normalized peak NMDA receptor currents showing the effect of PKI (0.2 µM, myristoylated) in striatal neurons isolated from WT and Cdk5 cKO mice. (b) Representative NMDA receptor current traces from data of panel a). (c) Cumulative data showing the percentage reduction of NMDA receptor currents by PKI in neurons from WT and Cdk5 cKO mice (n = 9–10). (d-f) Dot plots showing the NMDA receptor current amplitudes (d), capacitance (e), and current densities (f) in neurons from WT and Cdk5 cKO mice (n = 12). All data shown are means ± s.e.m., *P < 0.05, **P < 0.01, ***P < 0.001.
Supplementary Figure 5 Cdk5 cKO exhibit reduced spine density in medium spiny neurons and altered behavioral responses to stress.
(a) Dendritic spine density of medium spiny neurons in the ventral striatum is decreased in Cdk5 KO mice as compared to controls (n = 14–15 cells from four mice per genotype). (b) No change in spine types, such as thin, mushroom, stubby was observed between genotypes. (c) The effect of Cdk5 cKO on social defeat. Social avoidance was assessed by time spent interacting with the social interaction target. Graphs depict time spent in interaction zone (left) and corner zones (right) in present (Target) or absence (No Target) of interactor mouse (n = 7–13). (d) Assessment of cage activity in Cdk5 cKO and control mice (n = 13–14). (e) The effect of Cdk5 cKO on social interaction. Graphs depict time spent in interaction zone (left) and corner zones (right) in presence (Target) or absence (No Target) of interactor mouse (n = 17–24). All data shown are means ± s.e.m., *P < 0.05, **P < 0.01, ***P < 0.001.
Supplementary Figure 6 Forced swim test, social defeat and chronic unpredictable stress induce changes in PDE4 phosphorylation
(a,b) Quantitative immunoblot analysis of ventral striatum lysates from mice 1 h after FST (n = 4) compared to non-swim controls (n = 4) for pPDE4 (PKA) and pPDE4 (Cdk5). (c,d) Analysis of ventral striatum lysates from chronic socially defeated mice (n = 10) and non-defeated controls (n = 6) 1 h after social interaction testing for pPDE4 (PKA) and pPDE4 (Cdk5). (e,f) Analysis of ventral striatum lysates mice undergoing chronic unpredictable stress (n = 6) and non-stressed controls (n = 5) for pPDE4 (PKA) and pPDE4 (Cdk5). All data shown are means ± s.e.m., *P < 0.05, **P < 0.01, ***P < 0.001.
Supplementary Figure 7 Behavioral characterization of virus-mediated Cdk5 knockout in ventral striatum.
(a) Immunostain showing Cdk5 knockout in the ventral striatum induced by stereotactic bilateral infusion of AAV2-Cre into the NAc of homozygous floxed Cdk5 (fl/fl AAV) and WT mice (WT AAV). Arrowheads at the border of viral transduction fields indicate locations of inserts (right). (b) Assessment of cage activity in fl/fl AAV and WT AAV (n = 7–8). (c,d) Elevated plus maze performance of fl/fl AAV and WT AAV (n = 7–8) indicating time in zones (c) and locomotor activity (d). (e) Social interaction (SI) in fl/fl AAV and WT AAV (n = 10). All data shown are means ± s.e.m., *P < 0.05, **P < 0.01, ***P < 0.001.
Supplementary Figure 8 Reduced PDE4 phosphorylation in striatum of D1R-Cdk5-KO.
(a) Immunostain showing D1 dopamine receptor promoter-driven Cre expression via a GFP reporter (top) and Cdk5 loss in medium spiny neurons of ventral striatum (encircled; bottom). (b) Reduced Cdk5 expression in striatal lysates from D1R-Cdk5-KO (n = 6). (c,d) Quantitative immunoblot analysis of ventral striatum lysates from D1R-Cdk5-KO mice and controls (n = 6) for pPDE4 (PKA) (c) and (Cdk5) (d). All data shown are means ± s.e.m., *P < 0.05, **P < 0.01, ***P < 0.001.
Supplementary Figure 9 Cdk5 knockout in D1 dopamine receptor neurons alters stress-induced behavioral responses.
(a,b) D1R-Cdk5-KO exhibit reduced immobility and increased latency to initiation of floating in the FST (n = 9–16) (a) and increased time struggling in the TST (n = 12–14) (b). (c) Effect of D1R-Cdk5-KO on social defeat. Social avoidance was assessed by time spent interacting with the social target. Graphs depict time spent in interaction zone (left) and corner zones (right) in presence (Target) or absence (No Target) of interactor mouse (n = 8–10). (d) Locomotor activity during social interaction testing (n = 8–10). (e) Social interaction (SI) in D1R-Cdk5-KO (n = 10). (f,g) Elevated plus maze performance of D1R-Cdk5-KO and control mice (n = 7) indicating time in zones (f) and locomotor activity (g). (h) Assessment of cage activity in D1R-Cdk5-KO and control mice (n = 12–13). (i) Evaluation of locomotor activity and center times in the open field assay for D1R-Cdk5-KO and control mice (n = 9–10). All data shown are means ± s.e.m., *P < 0.05, **P < 0.01, ***P < 0.001.
Supplementary Figure 10 Increased water intake in D1R-Cdk5-KO mice.
(a) Effect of Cdk5 knockout in the D1R-Cdk5-KO mouse lines on sucrose preference test (SPT), a test of anhedonia that does not rely on locomotor activity. Graph depicts sucrose preference for D1R-Cdk5-KO (n = 10) and control mice (n = 15). (b) Total liquid consumption per day measured during water, sucrose and choice (water and sucrose) phase of the SPT. (c) Normalized intake volumes for each bottle during water and sucrose phase show no bias. (d) D1R-Cdk5-KO mice exhibit normal water intake during the night cycle, but have increased water intake during the day cycle (n = 5–6). All data shown are means ± s.e.m., *P < 0.05, **P < 0.01, ***P < 0.001.
Supplementary Figure 11 Reversal of forced swim test phenotype by PKA inhibition in D1R-Cdk5-KO and Cdk5 cKO.
(a,b) Effect of infusion of the PKA inhibitor Rp-cAMPs into ventral striatum of D1R-Cdk5-KO (a) and Cdk5 cKO (b) as assessed by the two-trial FST (n = 6–10). All data shown are means ± s.e.m., *P < 0.05, **P < 0.01, ***P < 0.001.
Supplementary Figure 12 In vitro and in vivo assessment of PDE4-siP specificity and its effect on PDE4 phosphorylation.
(a) Effect of the D1 dopamine receptor agonist SKF81297 (SKF), PDE4-siP, or both on the defined Cdk5 site, phospho-Thr75 DARPP-32 (pT75 D-32). †P < 0.05 compared to SKF81297 only; one-way ANOVA and Newman-Keuls test, (n = 4–8). (b) Evaluation of cage activity in mice infused with PDE4-siP (n = 5) and scrambled peptide (n = 9) before (40 min) and after (30 min) FST. (c) Effect of infusion of the PDE4 peptide with an N-terminal poly-arginine (R7) cell membrane permeablizing tag into NAc on the two-trial FST (n = 5–7). (d) Effect of infusion of peptides, in which the PKA (S133A; n = 8), Cdk5 (S145A; n = 8), or both serine sites (S133A/S145A; n = 8) were mutated to alanine, into NAc on the two-trial FST (n = 5–10) and compared to infusions with PDE4-siP (n = 9) and scrambled peptide (n = 7). (e) Infusion of the PDE4-siP into the ventral striatum has no impact on PDE4 phosphorylation levels at the Cdk5 and PKA sites within the dorsal striatum (n = 5–6). All data shown are means ± s.e.m., *P < 0.05, **P < 0.01, ***P < 0.001.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–13, and Supplementary Tables 1 and 2 (PDF 2722 kb)
Supplementary Methods Checklist
(PDF 496 kb)
Rights and permissions
About this article
Cite this article
Plattner, F., Hayashi, K., Hernández, A. et al. The role of ventral striatal cAMP signaling in stress-induced behaviors. Nat Neurosci 18, 1094–1100 (2015). https://doi.org/10.1038/nn.4066
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4066
This article is cited by
-
Cdk5 mediates rotational force-induced brain injury
Scientific Reports (2023)
-
A high-performance genetically encoded fluorescent indicator for in vivo cAMP imaging
Nature Communications (2022)
-
Neuroprotective effects of roflumilast against quinolinic acid-induced rat model of Huntington’s disease through inhibition of NF-κB mediated neuroinflammatory markers and activation of cAMP/CREB/BDNF signaling pathway
Inflammopharmacology (2021)
-
Homeostatic cAMP regulation by the RGS7 complex controls depression-related behaviors
Neuropsychopharmacology (2019)
-
The Neuroprotective Effect of L-Stepholidine on Methamphetamine-Induced Memory Deficits in Mice
Neurotoxicity Research (2019)