Abstract
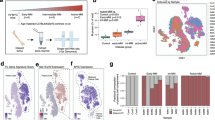
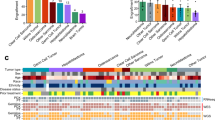
Most human cancers, including myeloma, are preceded by a precursor state. There is an unmet need for in vivo models to study the interaction of human preneoplastic cells in the bone marrow microenvironment with non-malignant cells. Here, we genetically humanized mice to permit the growth of primary human preneoplastic and malignant plasma cells together with non-malignant cells in vivo. Growth was largely restricted to the bone marrow, mirroring the pattern in patients with myeloma. Xenografts captured the genomic complexity of parental tumors and revealed additional somatic changes. Moreover, xenografts from patients with preneoplastic gammopathy showed progressive growth, suggesting that the clinical stability of these lesions may in part be due to growth controls extrinsic to tumor cells. These data demonstrate a new approach to investigate the entire spectrum of human plasma cell neoplasia and illustrate the utility of humanized models for understanding the functional diversity of human tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Palumbo, A. & Anderson, K. Multiple myeloma. N. Engl. J. Med. 364, 1046–1060 (2011).
Schüler, J., Ewerth, D., Waldschmidt, J., Wäsch, R. & Engelhardt, M. Preclinical models of multiple myeloma: a critical appraisal. Expert Opin. Biol. Ther. 13 (Suppl. 1), S111–S123 (2013).
Yaccoby, S., Barlogie, B. & Epstein, J. Primary myeloma cells growing in SCID-hu mice: a model for studying the biology and treatment of myeloma and its manifestations. Blood 92, 2908–2913 (1998).
Calimeri, T. et al. A unique three-dimensional SCID-polymeric scaffold (SCID-synth-hu) model for in vivo expansion of human primary multiple myeloma cells. Leukemia 25, 707–711 (2011).
Rongvaux, A. et al. Human hemato-lymphoid system mice: current use and future potential for medicine. Annu. Rev. Immunol. 31, 635–674 (2013).
Rongvaux, A. et al. Development and function of human innate immune cells in a humanized mouse model. Nat. Biotechnol. 32, 364–372 (2014).
Deng, K. et al. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature 517, 381–385 (2015).
Kishimoto, T. Interleukin-6: from basic science to medicine—40 years in immunology. Annu. Rev. Immunol. 23, 1–21 (2005).
Kishimoto, T., Akira, S. & Taga, T. Interleukin-6 and its receptor: a paradigm for cytokines. Science 258, 593–597 (1992).
Tassone, P. et al. A clinically relevant SCID-hu in vivo model of human multiple myeloma. Blood 106, 713–716 (2005).
Matsui, W. et al. Characterization of clonogenic multiple myeloma cells. Blood 103, 2332–2336 (2004).
Hosen, N. et al. CD138-negative clonogenic cells are plasma cells but not B cells in some multiple myeloma patients. Leukemia 26, 2135–2141 (2012).
Kim, D., Park, C.Y., Medeiros, B.C. & Weissman, I.L. CD19−CD45low/− CD38high/CD138+ plasma cells enrich for human tumorigenic myeloma cells. Leukemia 26, 2530–2537 (2012).
Kukreja, A. et al. Enhancement of clonogenicity of human multiple myeloma by dendritic cells. J. Exp. Med. 203, 1859–1865 (2006).
Klco, J.M. et al. Functional heterogeneity of genetically defined subclones in acute myeloid leukemia. Cancer Cell 25, 379–392 (2014).
Goyama, S., Wunderlich, M. & Mulloy, J.C. Xenograft models for normal and malignant stem cells. Blood 125, 2630–2640 (2015).
Zhao, S. et al. Serial exome analysis of disease progression in premalignant gammopathies. Leukemia 28, 1548–1552 (2014).
Hanamura, I. et al. Frequent gain of chromosome band 1q21 in plasma-cell dyscrasias detected by fluorescence in situ hybridization: incidence increases from MGUS to relapsed myeloma and is related to prognosis and disease progression following tandem stem-cell transplantation. Blood 108, 1724–1732 (2006).
Nair, S. et al. Clonal immunoglobulin against lysolipids in the origin of myeloma. N. Engl. J. Med. 374, 555–561 (2016).
Koduru, S. et al. Dendritic cell-mediated activation-induced cytidine deaminase (AID)-dependent induction of genomic instability in human myeloma. Blood 119, 2302–2309 (2012).
Dhodapkar, M.V. & Dhodapkar, K.M. Immune modulation in hematologic malignancies. Semin. Oncol. 42, 617–625 (2015).
Melchor, L. et al. Single-cell genetic analysis reveals the composition of initiating clones and phylogenetic patterns of branching and parallel evolution in myeloma. Leukemia 28, 1705–1715 (2014).
Ghobrial, I.M. Myeloma as a model for the process of metastasis: implications for therapy. Blood 120, 20–30 (2012).
Walker, B.A. et al. Intraclonal heterogeneity is a critical early event in the development of myeloma and precedes the development of clinical symptoms. Leukemia 28, 384–390 (2014).
López-Corral, L. et al. The progression from MGUS to smoldering myeloma and eventually to multiple myeloma involves a clonal expansion of genetically abnormal plasma cells. Clin. Cancer Res. 17, 1692–1700 (2011).
Dhodapkar, M.V. et al. Clinical, genomic, and imaging predictors of myeloma progression from asymptomatic monoclonal gammopathies (SWOG S0120). Blood 123, 78–85 (2014).
Dhodapkar, M.V. et al. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J. Exp. Med. 197, 1667–1676 (2003).
Dhodapkar, M.V., Krasovsky, J., Osman, K. & Geller, M.D. Vigorous premalignancy-specific effector T cell response in the bone marrow of patients with monoclonal gammopathy. J. Exp. Med. 198, 1753–1757 (2003).
Spisek, R. et al. Frequent and specific immunity to the embryonal stem cell-associated antigen SOX2 in patients with monoclonal gammopathy. J. Exp. Med. 204, 831–840 (2007).
Dhodapkar, M.V. et al. Prospective analysis of antigen-specific immunity, stem-cell antigens, and immune checkpoints in monoclonal gammopathy. Blood 126, 2475–2478 (2015).
Lawson, M.A. et al. Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche. Nat. Commun. 6, 8983 (2015).
Sehgal, K. et al. Clinical and pharmacodynamic analysis of pomalidomide dosing strategies in myeloma: impact of immune activation and cereblon targets. Blood 125, 4042–4051 (2015).
Zhao, S. et al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc. Natl. Acad. Sci. USA 110, 2916–2921 (2013).
Choi, M. et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 331, 768–772 (2011).
Acknowledgements
This work was supported by funds from the US National Institutes of Health (CA156689, CA106802, CA197603) and the clinical research priority program of the University of Zurich. The authors thank F. Giráldez-López, I. Tikhonova, S. Mane and M. Youngblood for assistance with sequencing analyses and C. Weibel, J. Alderman and C. Foster (all at Yale School of Medicine) for assistance with mouse colonies.
Author information
Authors and Affiliations
Contributions
M.V.D. and R.A.F. conceived and supervised the overall project, designed experiments and analyzed results. R.D., T.S. and R.V. designed and performed experiments and analyzed data. S.K., A.H., S.H., M.H.K., C.B., L.Z. and A.B. performed experiments and analyzed data. E.E. and M.G.M. designed experiments and analyzed data. M.V.D., R.D., T.S., R.V., M.G.M. and R.A.F. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text, Figures and Tables
Supplementary Figures 1–6 and Supplementary Tables 1–3 (PDF 29135 kb)
Rights and permissions
About this article
Cite this article
Das, R., Strowig, T., Verma, R. et al. Microenvironment-dependent growth of preneoplastic and malignant plasma cells in humanized mice. Nat Med 22, 1351–1357 (2016). https://doi.org/10.1038/nm.4202
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4202
This article is cited by
-
Humanized mouse models for immuno-oncology research
Nature Reviews Clinical Oncology (2023)
-
Mesenchymal stromal cell senescence in haematological malignancies
Cancer and Metastasis Reviews (2023)
-
Preclinical models for prediction of immunotherapy outcomes and immune evasion mechanisms in genetically heterogeneous multiple myeloma
Nature Medicine (2023)
-
Inhibitors of Bcl-2 and Bruton’s tyrosine kinase synergize to abrogate diffuse large B-cell lymphoma growth in vitro and in orthotopic xenotransplantation models
Leukemia (2022)
-
Calorie restriction has no effect on bone marrow tumour burden in a Vk*MYC transplant model of multiple myeloma
Scientific Reports (2022)