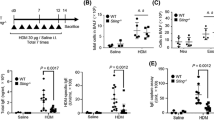
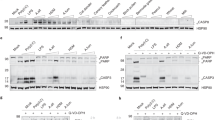
Abstract
Allergic asthma is a complex disease characterized by eosinophilic pulmonary inflammation, mucus production and reversible airway obstruction1. Exposure to indoor allergens is a risk factor for asthma, but this disease is also associated with high household levels of total and particularly Gram-negative bacteria2. The ability of bacterial products to act as adjuvants3 suggests they might promote asthma by priming allergic sensitization to inhaled allergens. In support of this idea, house dust extracts (HDEs) can activate antigen-presenting dendritic cells (DCs) in vitro and promote allergic sensitization to inhaled innocuous proteins in vivo4. It is unknown which microbial products provide most of the adjuvant activity in HDEs. A screen for adjuvant activity of microbial products revealed that the bacterial protein flagellin (FLA) stimulated strong allergic airway responses to an innocuous inhaled protein, ovalbumin (OVA). Moreover, Toll-like receptor 5 (TLR5), the mammalian receptor for FLA5,6, was required for priming strong allergic responses to natural indoor allergens present in HDEs. In addition, individuals with asthma have higher serum levels of FLA-specific antibodies as compared to nonasthmatic individuals. Together, these findings suggest that household FLA promotes the development of allergic asthma by TLR5-dependent priming of allergic responses to indoor allergens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Busse, W.W. & Lemanske, R.F. Jr. Asthma. N. Engl. J. Med. 344, 350–362 (2001).
Ross, M.A. et al. Association of asthma symptoms and severity with indoor bioaerosols. Allergy 55, 705–711 (2000).
Iwasaki, A. & Medzhitov, R. Regulation of adaptive immunity by the innate immune system. Science 327, 291–295 (2010).
Ng, N., Lam, D., Paulus, P., Batzer, G. & Horner, A.A. House dust extracts have both TH2 adjuvant and tolerogenic activities. J. Allergy Clin. Immunol. 117, 1074–1081 (2006).
Gewirtz, A.T., Navas, T.A., Lyons, S., Godowski, P.J. & Madara, J.L. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167, 1882–1885 (2001).
Hayashi, F. et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410, 1099–1103 (2001).
Barczyk, A., Pierzchala, W. & Sozanska, E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir. Med. 97, 726–733 (2003).
McKinley, L. et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J. Immunol. 181, 4089–4097 (2008).
Wilson, R.H. et al. Allergic sensitization through the airway primes TH17-dependent neutrophilia and airway hyperresponsiveness. Am. J. Respir. Crit. Care Med. 180, 720–730 (2009).
Franchi, L. et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in Salmonella-infected macrophages. Nat. Immunol. 7, 576–582 (2006).
Miao, E.A. et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat. Immunol. 7, 569–575 (2006).
Sung, S.S. et al. A major lung CD103 αE-β7 integrin–positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J. Immunol. 176, 2161–2172 (2006).
Sanders, C.J., Moore, D.A. III, Williams, I.R. & Gewirtz, A.T. Both radioresistant and hemopoietic cells promote innate and adaptive immune responses to flagellin. J. Immunol. 180, 7184–7192 (2008).
Pasare, C. & Medzhitov, R. Control of B-cell responses by Toll-like receptors. Nature 438, 364–368 (2005).
Janot, L. et al. Radioresistant cells expressing TLR5 control the respiratory epithelium's innate immune responses to flagellin. Eur. J. Immunol. 39, 1587–1596 (2009).
Mijares, L.A. et al. Airway epithelial MyD88 restores control of Pseudomonas aeruginosa murine infection via an IL-1–dependent pathway. J. Immunol. 186, 7080–7088 (2011).
Hammad, H. et al. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 15, 410–416 (2009).
McDonough, M.W. & Smith, S.E. Molecular weight variation among bacterial flagellins. Microbios 16, 49–53 (1976).
McKinley, L., Kim, J., Bolgos, G.L., Siddiqui, J. & Remick, D.G. Reproducibility of a novel model of murine asthma-like pulmonary inflammation. Clin. Exp. Immunol. 136, 224–231 (2004).
Braun-Fahrländer, C. et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N. Engl. J. Med. 347, 869–877 (2002).
El-Sharif, N., Douwes, J., Hoet, P. & Nemery, B. Childhood asthma and indoor aeroallergens and endotoxin in Palestine: a case-control study. J. Asthma 43, 241–247 (2006).
Gehring, U. et al. Exposure to house dust endotoxin and allergic sensitization in adults. Allergy 59, 946–952 (2004).
Bolte, G. et al. Early endotoxin exposure and atopy development in infants: results of a birth cohort study. Clin. Exp. Allergy 33, 770–776 (2003).
Thorne, P.S. et al. Endotoxin exposure is a risk factor for asthma: the national survey of endotoxin in United States housing. Am. J. Respir. Crit. Care Med. 172, 1371–1377 (2005).
Eisenbarth, S.C. et al. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J. Exp. Med. 196, 1645–1651 (2002).
Custovic, A. et al. Effect of day care attendance on sensitization and atopic wheezing differs by Toll-like receptor 2 genotype in 2 population-based birth cohort studies. J. Allergy Clin. Immunol. 127, 390–397 (2011).
Eder, W. et al. Opposite effects of CD14/-260 on serum IgE levels in children raised in different environments. J. Allergy Clin. Immunol. 116, 601–607 (2005).
Simpson, A. et al. Endotoxin exposure, CD14, and allergic disease: an interaction between genes and the environment. Am. J. Respir. Crit. Care Med. 174, 386–392 (2006).
Merx, S., Zimmer, W., Neumaier, M. & Ahmad-Nejad, P. Characterization and functional investigation of single nucleotide polymorphisms (SNPs) in the human TLR5 gene. Hum. Mutat. 27, 293 (2006).
Strindelius, L., Filler, M. & Sjoholm, I. Mucosal immunization with purified flagellin from Salmonella induces systemic and mucosal immune responses in C3H/HeJ mice. Vaccine 22, 3797–3808 (2004).
Wang, B.Z. et al. Incorporation of membrane-anchored flagellin into influenza virus-like particles enhances the breadth of immune responses. J. Virol. 82, 11813–11823 (2008).
Didierlaurent, A. et al. Flagellin promotes myeloid differentiation factor 88–dependent development of TH2-type response. J. Immunol. 172, 6922–6930 (2004).
Uematsu, S. et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat. Immunol. 9, 769–776 (2008).
Cunningham, A.F. et al. Responses to the soluble flagellar protein FliC are TH2, while those to FliC on Salmonella are TH1. Eur. J. Immunol. 34, 2986–2995 (2004).
Schulke, S. et al. A fusion protein of flagellin and ovalbumin suppresses the TH2 response and prevents murine intestinal allergy. J. Allergy Clin. Immunol. 128, 1340–1348 (2011).
Yousefi, S. et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 14, 949–953 (2008).
Karp, C.L. Guilt by intimate association: what makes an allergen an allergen? J. Allergy Clin. Immunol. 125, 955–960 (2010).
Trompette, A. et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 457, 585–588 (2009).
Whitehead, G.S. et al. The chemokine receptor D6 has opposing effects on allergic inflammation and airway reactivity. Am. J. Respir. Crit. Care. Med. 175, 243–249 (2007).
Lam, D., Ng, N., Lee, S., Batzer, G. & Horner, A.A. Airway house dust extract exposures modify allergen-induced airway hypersensitivity responses by TLR4-dependent and independent pathways. J. Immunol. 181, 2925–2932 (2008).
Sever, M.L. et al. Cockroach allergen reduction by cockroach control alone in low-income urban homes: a randomized control trial. J. Allergy Clin. Immunol. 120, 849–855 (2007).
Acknowledgements
This work was supported by the Intramural Research Program of the US National Institutes of Health NIEHS. We thank the human subjects for their blood donations and B. Yingling, J. Marshburn and A. Rice of the NIEHS Clinical Research Unit and D. Beaver of Duke University for assistance with patient recruitment and serum isolation. J. Hollingsworth (Duke University) with permission from R. Medzhitov (Yale University) provided CD11c-Myd88 transgenic mice on a Myd88−/− background. S. Akira (Osaka University) provided Tlr2−/−, Tlr4−/− and Myd88−/− mice and J. Ting (University of North Carolina) provided Nlrc4−/− mice. D. Wozniak (Ohio State University) provided both strains of P. aeruginosa, F.-S. Shu (Wayne State University) provided purified FLA from P. aeruginosa and A. Gewirtz (Emory University) provided FLA from Escherichia coli. We also thank NIEHS members K. Nakano for genotyping, L. Perrow for mouse colony management, N. Flagler for histology, J. Aloor for measuring endotoxin levels in HDEs, M. Sifre and C. Bortner for help with FACS-based cell sorting, J. Ciencewicki for advice on airway epithelial cell culture and M. Fessler and S. London for suggestions on the manuscript.
Author information
Authors and Affiliations
Contributions
R.H.W. and S.M. conducted the majority of the experiments, designed experiments and helped prepare the manuscript. G.S.W. performed some assays and mouse experiments, including most airway physiology experiments. D.C.Z. and M.L.S. provided dust samples from US households, and M.L.S. performed the Multiplex Array for Indoor Allergens. J.F.F. and G.P.F. analyzed and graded lung sections for mucus production and took photographs. H.N. helped with flow cytometry and experimental design. M.K. and S.G. provided sera from patients with asthma and control subjects. D.N.C. conceived of the project and helped with experimental design and writing of the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Table 1, Supplementary Figures 1–7 and Supplementary Methods (PDF 1843 kb)
Rights and permissions
About this article
Cite this article
Wilson, R., Maruoka, S., Whitehead, G. et al. The Toll-like receptor 5 ligand flagellin promotes asthma by priming allergic responses to indoor allergens. Nat Med 18, 1705–1710 (2012). https://doi.org/10.1038/nm.2920
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2920
This article is cited by
-
Emerging Insights into the Impact of Air Pollution on Immune-Mediated Asthma Pathogenesis
Current Allergy and Asthma Reports (2022)
-
Lung Function and Gene Expression of Pathogen Recognition Pathway Receptors: the Cardia Lung Study
Scientific Reports (2020)
-
The External Exposome and Food Allergy
Current Allergy and Asthma Reports (2020)
-
Role of Environmental Adjuvants in Asthma Development
Current Allergy and Asthma Reports (2020)
-
TLR5 Activation Exacerbates Airway Inflammation in Asthma
Lung (2020)