Abstract
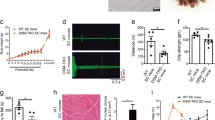
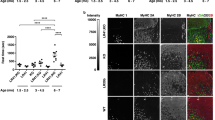
Myotonic dystrophy (DM) is commonly associated with CTG repeat expansions within the gene for DM–protein kinase (DMPK). The effect of altered expression levels of DMPK, which is ubiquitously expressed in all muscle cell lineages during development, was examined by disrupting the endogenous Dmpk gene and overexpressing a normal human DMPK transgene in mice. Nullizygous (−/−) mice showed only inconsistent and minor size changes in head and neck muscle fibres at older age, animals with the highest DMPK transgene expression showed hypertrophic cardiomyopathy and enhanced neonatal mortality. However, both models lack other frequent DM symptoms including the fibre–type dependent atrophy, myotonia, cataract and male–infertility. These results strengthen the contention that simple loss– or gain–of–expression of DMPK is not the only crucial requirement for development of the disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Harper, P.S. Myotonic dystrophy 2nd ed. (W.B. Saunders, London) (1989).
Brook, J.D. et al. Molecular basis of myotonic dystrophy: Expansion of a trinucleotide (CTG) repeat at the 3' end of a transcript encoding a protein kinase family member. Cell 68, 799–808 (1992).
Fu, Y.-H. et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science 255, 1256–1258 (1992).
Mahadevan, M. et al. Myotonic dystrophy mutation: An unstable CTG repeat in the 3′ untranslated region of the gene. Science 255, 1253–1255 (1992).
Hunter, A.G.W. et al. The correlation of age of onset wit CTG trinucleotide repeat amplification in myotonic dystrophy. J. Med. Genet. 29, 774–779 (1992).
Harley, H. et al. Size of the unstable CTG repeat sequence in relation to phenotype and parental transmission in myotonic dystrophy. Am. J. Hum. Genet. 52, 1164–1174 (1993).
Brunner, H.G. et al. Influence of sex of the transmitting parent as well as of parental allele size on the CTG expansion in myotonic dystrophy (DM). Am. J. Hum. Genet. 53, 1016–1023 (1993).
Jaspert, A., Fahsold, R., Grehl, H. & Claus, D. Myotonic dystrophy: correlation of clinical symptoms with the size of the CTG trinucleotide repeat. J.Neurol. 242, 99–104 (1995).
Jansen, G. et al. Characterization of the myotonic dystrophy region predicts multiple protein isoform-encoding mRNAs. Nature Genet. 1, 261–266 (1992).
Dunne, P.W., Walch, E.T. & Epstein, H.F. Phosphorylation reactions of recombinant human myotonic dystrophy protein kinase and their inhibition. Biochemistry 33, 10805–10814 (1994).
Wieringa, B. Myotonic dystrophy reviewed: Back to the future? Hum. Molec. Genet. 3, 1–7 (1994).
Jansen, G. et al. Gonosomal mosaicism in myotonic dystrophy patients: Involvement of mitotic events in (CTG)n repeat variation and selection against extreme expansion in sperm. Am. J. Hum. Genet. 54, 575–585 (1994).
Monckton, D.G., Wong, L.-J.C., Ashizawa, T. & Caskey, C.K. Somatic mosaicism, germline expansions, germline reversions and intergenerational reductions in myotonic dystrophy males: small pool PCR analyses. Hum. Mol. Genet. 4, 1–8 (1995).
Fu, Y.-H. et al. Decreased expression of myotonin-protein kinase messenger RNA and protein in adult form of myotonic dystrophy. Science 260, 235–238 (1993).
Hofmann-Radvanyi, H. et al. Myotonic dystrophy: Absence of CTG enlarged transcript in congenital forms, and low expression of the normal allele. Hum. Mol. Genet. 2, 1263–1267 (1993).
Koga, R. et al. Decreased myotonin-protein kinase in the skeletal and cardiac muscles in myotonic dystrophy. Biochem. Biophys. Res. Commun. 202, 577–585 (1994).
Sabourin, L.A. et al. Effect of the myotonic dystrophy (DM) mutation on mRNA levels of the DM gene. Nature Genet. 4, 233–238 (1993).
Krahe, R. et al. Effect of myotonic dystrophy trinucleotide repeat expansion on DMPK transcription and processing. Genomics 28, 1–14 (1995).
Jansen, G., Bächner, D., Coerwinkel, M., Wormskamp, N., Hameister, H. & Wieringa, B. Structural organization and developmental expression pattern of the DMR-N9 gene immediately upstream of the myotonic dystrophy locus. Hum. Mol. Genet 4, 843–852 (1995).
Shaw, D.J. et al. Genomic organization and transcriptional units at the myotonic dystrophy locus. Genomics 18, 673–679 (1993).
Boucher, C.A. et al. A novel homeodomain-encoding gene is associated with a large CpG island interrupted by the myotonic dystrophy unstable (CTG)n repeat. Hum. Mol. Genet. 4, 1919–1925 (1995).
Van der Ven, P.F. M. et al. Myotonic dystrophy kinase is a component of neuromuscular junctions. Hum. Mol. Genet. 2, 1889–1894 (1993).
Whiting, E.J. et al. Characterization of myotonic dystrophy kinase (DMK) protein in human and rodent muscle and central nervous tissue. Hum. Mol. Genet. 4, 1063–1072 (1995).
Maeda, M. et al. Identification, tissue-specific expression and subcellular localization of the 80- and 71 kDa forms of myotonic dystrophy kinase protein. J. Biol. Chem. 270, 20246–20249 (1995).
Brewster, B.S., Jeal, S. & RN.Identification of a protein product of the myotonic dystrophy gene using peptide specific antibodies. Biochem. Biophys. Res. Commun. 194, 1256–1260 (1993).
Hämäläinen, N. & Pette, D. The histochemical profiles of fast fibre types MB, IID and IIA in skeletal muscles of mouse, rat and rabbit. J. Histochem. Cytochem. 41, 733–743 (1993).
Brunner, H.G., Hamel, B.C.J., Rieu, P., Höweler, C.J. & Peters, F.T.M. Intestinal pseudo-obstruction in myotonic dystrophy. J. Med. Genet. 29, 791–793 (1992).
Lem, J. et al. Retinal degeneration is rescued in transgenic rd mice by exrpressin of the cGMP phosphodiesterase beta subunit. Proc. Natl. Acad. Sci. USA. 89, 4422–4426 (1992).
Weiss, L.M., Movahed, L.A., Billingham, M.E. & Cleary, M.L. Detection of Coxsackievirus B3 RNA in myocardial tissues by the polymerase chain reaction. Am. J. Pathol. 138, 497–503 (1991).
Moorman, J.R. et al. Cardiac Involvement in myotonic dystrophy. Medicine 64, 371–378 (1985).
Nguyen, H.H. Wolfe III, J. T., Holmes, D.R. & Edwards, W.D. Pathology of the cardiac conduction system in myotonic dystrophy: a study of 12 cases. J. Am. Coll. Cardiol. 11, 662–671 (1988).
Melacini, P. et al. The natural history of cardiac involvement in myotonic dystrophy: an eight-year follow-up in 17 patients. Clin. Cardiol. 11, 231–238 (1988).
Van Echteld, C.J.A., Kirkels, J.H., Eijgelshoven, M.H.J., van der Meer, P. & Ruigrok, T.J.C. Intracellular sodium during ischemia and calcium-free perfusion: a 23Na NMR study. J. Mol. Cell. Cardiol. 23, 297–307 (1991).
Mahadevan, M.S. et al. Structure and genomic sequence of the myotonic dystrophy (DM kinase) gene. Hum. Molec. Genet 2, 299–304 (1993).
Taneja, K.L., McCurrach, M., Schalling, M., Housman, D. & Singer, R.H. Foci of trinucleotide repeat transcripts in nuclei of myotonic dystrophy cells and tissues. J. Cell Biol. 128, 995–1002 (1995).
Wang, Y.-H., Amirhaeri, S., Kang, S., Wells, R.D & Griffith, J.D. Preferential nucleosome assembly at DMA triplet repeats from the myotonic dystrophy gene. Science 265, 669–671 (1994).
Brilliant, M.H., Williams, R.W., Conti, C.J., Angel, J.M., Oakey, R.J. & Hol-dener, B.C., Chromosome 7. Mammal. Genet. 5, S104–S123 (1994).
Schiaffino, S. et al. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J. Muscle Res. Cell. Motil. 10, 197 (1989).
Beilharz, M.W., Lareu, R.R., Garrett, K.L., Grounds, M.D. & Fletcher, S. Quantitation of muscle precursor cell activity in skeletal muscle by northern analysis of MyoD and myogenin expression: application to dystrophic (mdx) mouse muscle. Mol. Cell. Neurosci. 3, 326–331 (1992).
Dangain, J. & Vrbova, G. Muscle development in mdx mutant mice. Muscle Nerve 7, 700–704 (1984).
Van Deursen, J. et al. Skeletal muscles of mice deficient in muscle creatine kinase lack burst activity. Cell 74, 621–631 (1993).
Van Deursen, J. & Wieringa, B. Targeting of the creatine kinase M gene in embryonic stem cells using isogenic and nonisogenic vectors. Nucl. Acids Res. 20, 3815–3820 (1992).
Bradley, A. Production and analysis of chimeric mice, in Teratocarcinomas and embryonic stem cells: A practical approach. 113–115 (IRLEd. Robertson, E.J. Press, Oxford, UK, 1987).
Larid, P.W. et al. Simplified mammalian DNA isolation procedure. Nucl. Acid. Res. 19, 4293 (1991).
Aslanidis, C. et al. Cloning of the essential myotonic dystrophy region and mapping of the putative defect. Nature 355, 548–551 (1992).
Tautz, D. & Pfeiffle, C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma 2, 81–85 (1989).
Rosen, B. & Beddington, R.S. Whole-mount in situ hybridization in the mouse embryo: gene expression in three dimensions. Trends Genef. 5, 162–167 (1993).
Bächner, D. et al. Enhanced expression of the FMR1 gene during germ cell proliferation suggests a special function in both the male and the female gonad. Hum. Mol. Genet. 2, 2043–2050 (1993).
Bianchi, G., Ferretti, P., Recchia, M., Rocchetti, M., Tavani, A. & Manara, L. Morphine tissue levels and reduction of gastrointestinal transit in rats. Correlation supports primary action site in the gut. Gastroenterology 85, 852–858 (1983).
Plomp, J.J., van Kempen, G.Th.H. & Molenaar, P.C. The upregulation of acetylcholine release at endplates of bungarotoxin-treated rats: Its dependency on calcium. J. Physiol 478, 125–136 (1994).
Van der Ven, P.F.M. PhD. Thesis (University of Nijmegen, Medical Faculty, The Netherlands, 1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jansen, G., Groenen, P., Bächner, D. et al. Abnormal myotonic dystrophy protein kinase levels produce only mild myopathy in mice. Nat Genet 13, 316–324 (1996). https://doi.org/10.1038/ng0796-316
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0796-316
This article is cited by
-
Transcriptomic profiles of muscular dystrophy with myositis (mdm) in extensor digitorum longus, psoas, and soleus muscles from mice
BMC Genomics (2022)
-
Comparative analysis of the transcriptomes of EDL, psoas, and soleus muscles from mice
BMC Genomics (2020)
-
Dosage effect of multiple genes accounts for multisystem disorder of myotonic dystrophy type 1
Cell Research (2020)
-
Nebulin nemaline myopathy recapitulated in a compound heterozygous mouse model with both a missense and a nonsense mutation in Neb
Acta Neuropathologica Communications (2020)
-
Myotonic dystrophy type 2 and modifier genes: an update on clinical and pathomolecular aspects
Neurological Sciences (2017)