Abstract
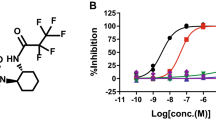
Pathological hyperphosphorylation of the microtubule-associated protein tau is characteristic of Alzheimer's disease (AD) and the associated tauopathies. The reciprocal relationship between phosphorylation and O-GlcNAc modification of tau and reductions in O-GlcNAc levels on tau in AD brain offers motivation for the generation of potent and selective inhibitors that can effectively enhance O-GlcNAc in vertebrate brain. We describe the rational design and synthesis of such an inhibitor (thiamet-G, Ki = 21 nM; 1) of human O-GlcNAcase. Thiamet-G decreased phosphorylation of tau in PC-12 cells at pathologically relevant sites including Thr231 and Ser396. Thiamet-G also efficiently reduced phosphorylation of tau at Thr231, Ser396 and Ser422 in both rat cortex and hippocampus, which reveals the rapid and dynamic relationship between O-GlcNAc and phosphorylation of tau in vivo. We anticipate that thiamet-G will find wide use in probing the functional role of O-GlcNAc in vertebrate brain, and it may also offer a route to blocking pathological hyperphosphorylation of tau in AD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Braak, H., Braak, E., Grundke-Iqbal, I. & Iqbal, K. Occurrence of neuropil threads in the senile human brain and in Alzheimer's disease: a third location of paired helical filaments outside of neurofibrillary tangles and neuritic plaques. Neurosci. Lett. 65, 351–355 (1986).
Lee, V.M., Goedert, M. & Trojanowski, J.Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 24, 1121–1159 (2001).
Ksiezak-Reding, H., Liu, W.K. & Yen, S.H. Phosphate analysis and dephosphorylation of modified tau associated with paired helical filaments. Brain Res. 597, 209–219 (1992).
Schneider, A., Biernat, J., von Bergen, M., Mandelkow, E. & Mandelkow, E.M. Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments. Biochemistry 38, 3549–3558 (1999).
Sengupta, A. et al. Phosphorylation of tau at both Thr 231 and Ser 262 is required for maximal inhibition of its binding to microtubules. Arch. Biochem. Biophys. 357, 299–309 (1998).
Lindwall, G. & Cole, R.D. Phosphorylation affects the ability of tau protein to promote microtubule assembly. J. Biol. Chem. 259, 5301–5305 (1984).
Arnold, C.S. et al. The microtubule-associated protein tau is extensively modified with O-linked N-acetylglucosamine. J. Biol. Chem. 271, 28741–28744 (1996).
Liu, F., Iqbal, K., Grundke-Iqbal, I., Hart, G.W. & Gong, C.X. O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer's disease. Proc. Natl. Acad. Sci. USA 101, 10804–10809 (2004).
Torres, C.R. & Hart, G.W. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J. Biol. Chem. 259, 3308–3317 (1984).
Cheng, X., Cole, R.N., Zaia, J. & Hart, G.W. Alternative O-glycosylation/O-phosphorylation of the murine estrogen receptor beta. Biochemistry 39, 11609–11620 (2000).
Chou, T.Y., Hart, G.W. & Dang, C.V. c-Myc is glycosylated at threonine 58, a known phosphorylation site and a mutational hot spot in lymphomas. J. Biol. Chem. 270, 18961–18965 (1995).
Comer, F.I. & Hart, G.W. Reciprocity between O-GlcNAc and O-phosphate on the carboxyl terminal domain of RNA polymerase II. Biochemistry 40, 7845–7852 (2001).
Zachara, N.E. & Hart, G.W. Cell signaling, the essential role of O-GlcNAc! Biochim. Biophys. Acta 1761, 599–617 (2006).
Kreppel, L.K., Blomberg, M.A. & Hart, G.W. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J. Biol. Chem. 272, 9308–9315 (1997).
Lubas, W.A., Frank, D.W., Krause, M. & Hanover, J.A. O-Linked GlcNAc transferase is a conserved nucleocytoplasmic protein containing tetratricopeptide repeats. J. Biol. Chem. 272, 9316–9324 (1997).
Dong, D.L. & Hart, G.W. Purification and characterization of an O-GlcNAc selective N-acetyl-β-D-glucosaminidase from rat spleen cytosol. J. Biol. Chem. 269, 19321–19330 (1994).
Gao, Y., Wells, L., Comer, F.I., Parker, G.J. & Hart, G.W. Dynamic O-glycosylation of nuclear and cytosolic proteins: cloning and characterization of a neutral, cytosolic β-N-acetylglucosaminidase from human brain. J. Biol. Chem. 276, 9838–9845 (2001).
Lefebvre, T. et al. Evidence of a balance between phosphorylation and O-GlcNAc glycosylation of Tau proteins–a role in nuclear localization. Biochim. Biophys. Acta 1619, 167–176 (2003).
Li, X., Lu, F., Wang, J.Z. & Gong, C.X. Concurrent alterations of O-GlcNAcylation and phosphorylation of tau in mouse brains during fasting. Eur. J. Neurosci. 23, 2078–2086 (2006).
Farook, V.S., Bogardus, C. & Prochazka, M. Analysis of MGEA5 on 10q24.1-q24.3 encoding the β-O-linked N-acetylglucosaminidase as a candidate gene for type 2 diabetes mellitus in Pima Indians. Mol. Genet. Metab. 77, 189–193 (2002).
Le Corre, S. et al. An inhibitor of tau hyperphosphorylation prevents severe motor impairments in tau transgenic mice. Proc. Natl. Acad. Sci. USA 103, 9673–9678 (2006).
Engel, T., Goni-Oliver, P., Lucas, J.J., Avila, J. & Hernandez, F. Chronic lithium administration to FTDP-17 tau and GSK-3β overexpressing mice prevents tau hyperphosphorylation and neurofibrillary tangle formation, but pre-formed neurofibrillary tangles do not revert. J. Neurochem. 99, 1445–1455 (2006).
Mazanetz, M.P. & Fischer, P.M. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat. Rev. Drug Discov. 6, 464–479 (2007).
Macauley, M.S., Whitworth, G.E., Debowski, A.W., Chin, D. & Vocadlo, D.J. O-GlcNAcase uses substrate-assisted catalysis: kinetic analysis and development of highly selective mechanism-inspired inhibitors. J. Biol. Chem. 280, 25313–25322 (2005).
Dorfmueller, H.C. et al. GlcNAcstatin: a picomolar, selective O-GlcNAcase inhibitor that modulates intracellular O-GlcNAcylation levels. J. Am. Chem. Soc. 128, 16484–16485 (2006).
Kim, E.J., Perreira, M., Thomas, C.J. & Hanover, J.A. An O-GlcNAcase-specific inhibitor and substrate engineered by the extension of the N-acetyl moiety. J. Am. Chem. Soc. 128, 4234–4235 (2006).
Knapp, S. et al. Tautomeric modification of GlcNAc-thiazoline. Org. Lett. 9, 2321–2324 (2007).
Shanmugasundaram, B. et al. Inhibition of O-GlcNAcase by a gluco-configured nagstatin and a PUGNAc-imidazole hybrid inhibitor. Chem. Commun. (Camb.) 4372–4374 (2006).
Stubbs, K.A., Zhang, N. & Vocadlo, D.J. A divergent synthesis of 2-acyl derivatives of PUGNAc yields selective inhibitors of O-GlcNAcase. Org. Biomol. Chem. 4, 839–845 (2006).
Haltiwanger, R.S., Grove, K. & Philipsberg, G.A. Modulation of O-linked N-acetylglucosamine levels on nuclear and cytoplasmic proteins in vivo using the peptide O-GlcNAc-β-N-acetylglucosaminidase inhibitor O-(2-acetamido-2-deoxy-D-glucopyranosylidene)amino-N-phenylcarbamate. J. Biol. Chem. 273, 3611–3617 (1998).
Horsch, M., Hoesch, L., Vasella, A. & Rast, D.M. N-acetylglucosaminono-1,5-lactone oxime and the corresponding (phenylcarbamoyl)oxime. Novel and potent inhibitors of β-N-acetylglucosaminidase. Eur. J. Biochem. 197, 815–818 (1991).
Scaffidi, A. et al. A 1-acetamido derivative of 6-epi-valienamine: an inhibitor of a diverse group of β-N-acetylglucosaminidases. Org. Biomol. Chem. 5, 3013–3019 (2007).
Zou, L. et al. The protective effects of PUGNAc on cardiac function after trauma-hemorrhage are mediated via increased protein O-GlcNAc levels. Shock 27, 402–408 (2007).
Macauley, M.S., Stubbs, K.A. & Vocadlo, D.J. O-GlcNAcase catalyzes cleavage of thioglycosides without general acid catalysis. J. Am. Chem. Soc. 127, 17202–17203 (2005).
Knapp, S. et al. NAG-thiazoline, An N-acetyl-β-hexosaminidase inhibitor that implicates acetamido participation. J. Am. Chem. Soc. 118, 6804–6805 (1996).
Whitworth, G.E. et al. Analysis of PUGNAc and NAG-thiazoline as transition state analogues for human O-GlcNAcase: mechanistic and structural insights into inhibitor selectivity and transition state poise. J. Am. Chem. Soc. 129, 635–644 (2007).
Cetinbas, N., Macauley, M.S., Stubbs, K.A., Drapala, R. & Vocadlo, D.J. Identification of Asp174 and Asp175 as the key catalytic residues of human O-GlcNAcase by functional analysis of site-directed mutants. Biochemistry 45, 3835–3844 (2006).
Knapp, S., Kirk, B., Vocadlo, D. & Withers, S. An allosamizoline/glucosamine hybrid NAGase inhibitor. Synlett 1997, 435–436 (1997).
Terwisscha van Scheltinga, A.C. et al. Stereochemistry of chitin hydrolysis by a plant chitinase/lysozyme and X-ray structure of a complex with allosamidin: evidence for substrate assisted catalysis. Biochemistry 34, 15619–15623 (1995).
Dennis, R.J. et al. Structure and mechanism of a bacterial β-glucosaminidase having O-GlcNAcase activity. Nat. Struct. Mol. Biol. 13, 365–371 (2006).
Greene, L.A. & Tischler, A.S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA 73, 2424–2428 (1976).
Comer, F.I., Vosseller, K., Wells, L., Accavitti, M.A. & Hart, G.W. Characterization of a mouse monoclonal antibody specific for O-linked N-acetylglucosamine. Anal. Biochem. 293, 169–177 (2001).
Li, T., Hawkes, C., Qureshi, H.Y., Kar, S. & Paudel, H.K. Cyclin-dependent protein kinase 5 primes microtubule-associated protein Tau site-specifically for glycogen synthase kinase 3β. Biochemistry 45, 3134–3145 (2006).
Cho, J.H. & Johnson, G.V. Primed phosphorylation of tau at Thr231 by glycogen synthase kinase 3β (GSK3β) plays a critical role in regulating tau's ability to bind and stabilize microtubules. J. Neurochem. 88, 349–358 (2004).
Kasai, K. & Yamaji, N. Novel chromogenic substrates for the rate-assay of N-acetyl-β-D-glucosaminidase-resorufinyl-N-acetyl and resazurinyl-N-acetyl-β-D-glucosaminides. Anal. Sci. 8, 161–164 (1992).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the maximum likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Acknowledgements
We thank the staff at the Animal Care Facility at Simon Fraser University and Y. Deng for assistance with the animal studies. T. Gloster is thanked for assistance with the X-ray structural studies. We also thank the Canadian Institute for Health Research (CIHR), the Scottish Rite Charitable Foundation (SRCF) and the Biotechnology and Biological Sciences Research Council (BBSRC) for providing funding for this work. M.S.M. and X.S. are recipients of senior scholarships from the Michael Smith Foundation for Health Research (MSFHR). X.S. is a doctoral research scholarship holder from the CIHR and the ALS Society of Canada. M.S.M. is also a scholarship holder from the Natural Science and Engineering Research Council of Canada (NSERC). Y.H. is the recipient of University of York Wild Fund support. G.J.D. is a Royal Society-Wolfson Research Merit Award recipient. D.J.V. is a scholar of the MSFHR and the Canada Research Chair in Chemical Glycobiology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that patent applications relevant to this work have been filed and that they are bound by confidentiality agreements preventing them from disclosing existing financial interests in this work.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Tables 1 and 2, and Supplementary Methods (PDF 2406 kb)
Rights and permissions
About this article
Cite this article
Yuzwa, S., Macauley, M., Heinonen, J. et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol 4, 483–490 (2008). https://doi.org/10.1038/nchembio.96
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.96
This article is cited by
-
Hyperglycemia and O-GlcNAc transferase activity drive a cancer stem cell pathway in triple-negative breast cancer
Cancer Cell International (2023)
-
O-GlcNAcylation determines the translational regulation and phase separation of YTHDF proteins
Nature Cell Biology (2023)
-
O-GlcNAcylation regulates neurofilament-light assembly and function and is perturbed by Charcot-Marie-Tooth disease mutations
Nature Communications (2023)
-
Chromatin-associated OGT promotes the malignant progression of hepatocellular carcinoma by activating ZNF263
Oncogene (2023)
-
Castration-resistant prostate cancer cells are dependent on the high activity of CDK7
Journal of Cancer Research and Clinical Oncology (2023)