Abstract
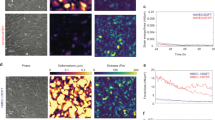
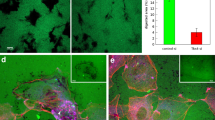
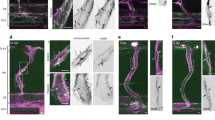
Endothelial cell (EC) movement is an initiating and rate-limiting event in the neogenesis and repair of blood vessels. Here, we explore the hypothesis that microviscosity of the plasma membrane (PM) is a key physiological regulator of cell movement. Aortic ECs treated with membrane-active agents, such as α-tocopherol, cholesterol and lysophospholipids, exhibited a biphasic dependency on membrane microviscosity, in which moderate increases enhanced EC migration, but increases beyond a threshold markedly inhibited migration. Surprisingly, angiogenic growth factors, that is, basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF), also increased membrane microviscosity, as measured in live cells by fluorescence recovery after photobleaching (FRAP). The localization of Rac to the PM was modified in cells treated with membrane-active agents or growth factors, suggesting a molecular mechanism for how membrane microviscosity influences cell movement. Our data show that angiogenic growth factors, as well as certain lipophilic molecules, regulate cell motility through alterations in membrane properties and the consequent relocalization of critical signalling molecules to membranes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Horwitz, A. R. & Parsons, J. T. Science 286, 1102–1103 (1999).
Lauffenburger, D. A. & Horwitz, A. F. Cell 84, 359–369 (1996).
Zetter, B. R. Annu. Rev. Med. 49, 407–424 (1998).
Murugesan, G., Chisolm, G. M. & Fox, P. L. J. Cell Biol. 120, 1011–1019 (1993).
Murugesan, G. & Fox, P. L. J. Clin. Invest. 97, 2736–2744 (1996).
Sheetz, M. P., Painter, R. G. & Singer, S. J. J. Cell Biol. 70, 193–203 (1976).
Kugiyama, K. et al. Atherosclerosis 143, 201–204 (1999).
Urano, S. et al. J. Biol. Chem. 267, 18365–18370 (1992).
Pryor, W. A. Free Radic. Biol. Med. 28, 141–164 (2000).
Yeagle, P. L., Albert, A. D., Boesze-Battaglia, K., Young, J. & Frye, J. Biophys. J. 57, 413–424 (1990).
Stillwell, W., Dallman, T., Dumaual, A. C., Crump, F. T. & Jenski, L. J. Biochemistry 35, 13353–13362 (1996).
Chang, H. M., Reitstetter, R. & Gruener, R. J. Membr. Biol. 145, 13–19 (1995).
Lundbæk, J. A. & Andersen, O. S. J. Gen. Physiol. 104, 645–673 (1994).
Van Corven, E. J., Groenink, A., Jalink, K., Eichholtz, T. & Moolenaar, W. H. Cell 59, 45–54 (1989).
Axelrod, D., Koppel, D. E., Schlessinger, J., Elson, E. & Webb, W. W. Biophys. J. 16, 1055–1069 (1976).
Nobes, C. D. & Hall, A. Cell 81, 53–62 (1995).
Bretscher, M. S. Cell 85, 465–467 (1996).
Felder, S. & Elson, E. L. J. Cell Biol. 111, 2513–2526 (1990).
Raucher, D. & Sheetz, M. P. J. Cell Biol. 148, 127–136 (2000).
Marom, M., Ben-Baruch, G., Roitelman, J. & Kloog, Y. Cell Mol. Neurobiol. 14, 119–132 (1994).
Scheiffele, P., Rietveld, A., Wilk, T. & Simons, K. J. Biol. Chem. 274, 2038–2044 (1999).
Pralle, A., Keller, P., Florin, E. L., Simons, K. & Hörber, J. K. J. Cell Biol. 148, 997–1008 (2000).
Gómez-Moutón, C. et al. Proc. Natl Acad. Sci USA 98, 9642–9647 (2001).
Isshiki, M. et al. J. Cell Sci. 115, 475–484 (2002).
Del Pozo, M. A., Price, L. S., Alderson, N. B., Ren, X. D. & Schwartz, M. A. EMBO J. 19, 2008–2014 (2000).
Chatterjee, S., Cheung, H. C. & Hunter, E. Proc. Natl Acad. Sci. USA 79, 835–839 (1982).
Shklar, G. & Schwartz, J. L. Eur. J. Cancer 32B, 114–119 (1996).
Butkerait, P. et al. J. Biol. Chem. 270, 18691–18699 (1995).
Sommer, A. et al. J. Biol. Chem. 267, 24217–24222 (1992).
Smart, E. J., Ying, Y. S., Mineo, C. & Anderson, R. G. Proc. Natl Acad. Sci. USA 92, 10104–10108 (1995).
Acknowledgements
We thank B. Anand-Apte for helpful discussions. This work was supported by National Institutes of Health grants HL/CA54519 and HL29582 to P.L.F. and HL64357 to L.M.G., National Aeronautics and Space Administration grant 96-HEDS-04 to P.L.F. and a Fellowship from the American Heart Association, Ohio Valley Affiliate to P.K.G.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ghosh, P., Vasanji, A., Murugesan, G. et al. Membrane microviscosity regulates endothelial cell motility. Nat Cell Biol 4, 894–900 (2002). https://doi.org/10.1038/ncb873
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb873
This article is cited by
-
Ultrafast viscosity measurement with ballistic optical tweezers
Nature Photonics (2021)
-
Imatinib modulates pro-inflammatory microenvironment with angiostatic effects in experimental lung carcinogenesis
Inflammopharmacology (2020)
-
Stimulus-dependent phosphorylation of profilin-1 in angiogenesis
Nature Cell Biology (2012)
-
Novel role of cPLA2α in membrane and actin dynamics
Cellular and Molecular Life Sciences (2010)
-
Coherent anti-Stokes Raman scattering imaging of lipids in cancer metastasis
BMC Cancer (2009)