Abstract
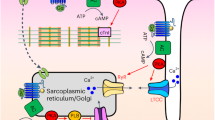
Stimulation of phosphoinositide-hydrolysing phospholipase C (PLC) generating inositol-1,4,5-trisphosphate is a major calcium signalling pathway used by a wide variety of membrane receptors, activating distinct PLC-β or PLC-γ isoforms1,2,3,4. Here we report a new PLC and calcium signalling pathway that is triggered by cyclic AMP (cAMP) and mediated by a small GTPase of the Rap family. Activation of the adenylyl cyclase-coupled β2-adrenoceptor expressed in HEK-293 cells or the endogenous receptor for prostaglandin E1 in N1E-115 neuroblastoma cells induced calcium mobilization and PLC stimulation, seemingly caused by cAMP formation, but was independent of protein kinase A (PKA). We provide evidence that these receptor responses are mediated by a Rap GTPase, specifically Rap2B, activated by a guanine-nucleotide-exchange factor (Epac) regulated by cAMP5,6, and involve the recently identified PLC-ɛ isoform7,8,9.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Berridge, M. J. & Irvine, R. F. Nature 341, 197–205 (1989).
Berridge, M. J., Lipp, P. & Bootman, M. D. Nature Rev. Mol. Cell Biol. 1, 11–21 (2000).
Exton, J. H. Annu. Rev. Pharmacol. Toxicol. 36, 481–509 (1996).
Rhee, S. G. & Bae, Y. S. J. Biol. Chem. 272, 15045–15048 (1997).
De Rooij, J. et al. Nature 396, 474–477 (1998).
De Rooij, J. et al. J. Biol. Chem. 275, 20829–20836 (2000).
Lopez, I., Mak, E. C., Ding, J., Hamm, H. E. & Lomasney, J. W. J. Biol. Chem. 276, 2758–2765 (2001).
Kelley, G. G., Reks, S. E., Ondrako, J. M. & Smrcka, A. V. EMBO J. 20, 743–754 (2001).
Song, C. et al. J. Biol. Chem. 276, 2752–2757 (2001).
Park, D. J., Min, H. K. & Rhee, S. G. J. Biol. Chem. 267, 1496–1501 (1992).
Liu, M. & Simon, M. I. Nature 382, 83–87 (1996).
Meyer zu Heringdorf, D. et al. EMBO J. 17, 2830–2837 (1998).
Schmidt, M. et al. J. Biol. Chem. 275, 32603–32610 (2000).
Daaka, Y., Luttrell, L. M. & Lefkowitz, R. J. Nature 390, 88–91 (1997).
Hanoune, J. & Defer, N. Annu. Rev. Pharmacol. Toxicol. 41, 145–174 (2001).
Walsh, D. A. & van Patten, S. M. FASEB J. 8, 1227–1236 (1994).
Chijiwa, T. et al. J. Biol. Chem. 265, 5267–5272 (1990).
Schmidt, M. et al. J. Biol. Chem. 273, 7413–7422 (1998).
Just, I. et al. Nature 375, 500–503 (1995).
Voβ, M. et al. J. Biol. Chem. 274, 34691–34698 (1999).
Murthy, S. N. P., Lomasney, J. W., Mak, E. C. & Lorand, L. Proc. Natl Acad. Sci. USA 96, 11815–11819 (1999).
Kim, Y.-H. et al. J. Biol. Chem. 274, 26127–26134 (1999).
Matsuzawa, H. & Nirenberg, M. Proc. Natl Acad. Sci. USA 72, 3472–3476 (1975).
Kanba, S. et al. J. Neurochem. 57, 2011–2015 (1991).
Missale, C., Nash, R., Robinson, S. W., Jaber, M. & Caron M. G. Physiol. Rev. 78, 189–225 (1998).
de la Peña, P., del Camino, D., Prado, L. A., Domínguez, P. & Barros, F. J. Biol. Chem. 270, 3554–3559 (1995).
Lin, C. W. et al. Mol. Pharmacol. 47, 131–139 (1995).
Chik, C. L. et al. J. Neurochem. 67, 1005–1013 (1996).
Daniel, P. B., Kieffer, T. J., Leech, C. A. & Habener, J. F. J. Biol. Chem. 276, 12938–12944 (2001).
Bos, J. L. EMBO J. 17, 6776–6782 (1998).
Bos, J. L., de Rooij, J. & Reedquist, K. A. Nature Rev. Mol. Cell Biol. 2, 369–377 (2001).
Evellin, S. et al. Naunyn-Schmiedeberg's Arch. Pharmacol. 363, R61 (2001).
Zhang, C., Schmidt, M., von Eichel-Streiber, C. & Jakobs, K. H. Mol. Pharmacol. 50, 864–869 (1996).
Schmidt, M. et al. Naunyn-Schmiedeberg's Arch. Pharmacol. 354, 87–94 (1996).
Van den Berghe, N., Cool, R. H., Horn, G. & Wittinghofer, A. Oncogene 15, 845–850 (1997).
Acknowledgements
We thank K. Baden, M. Hagedorn, H. Geldermann, D. Petermeyer, M. Michel and A. Rueppel for expert technical assistance, and R. Jockers, J. de Gunzburg, J. L. Bos, A. Wittinghofer and C. von Eichel-Streiber for providing various DNA constructs and proteins. This work was supported by the Deutsche Forschungsgemeinschaft, the Interne Forschungsförderung Essen (IFORES), the Fonds der Chemischen Industrie and the Council of Earth and Life Sciences and Chemical Sciences of The Netherlands Organisation for Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Figure S1
PGE1- and forskolin-induced PLC stimulation in N1E-115 neuroblastoma cells: role of cAMP, PKA, Epac1, Rap2B and PLC-ɛ (PDF 44 kb)
Rights and permissions
About this article
Cite this article
Schmidt, M., Evellin, S., Weernink, P. et al. A new phospholipase-C–calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat Cell Biol 3, 1020–1024 (2001). https://doi.org/10.1038/ncb1101-1020
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1101-1020
This article is cited by
-
cAMP activates calcium signalling via phospholipase C to regulate cellulase production in the filamentous fungus Trichoderma reesei
Biotechnology for Biofuels (2021)
-
Physiological effects of nutrients on insulin release by pancreatic beta cells
Molecular and Cellular Biochemistry (2021)
-
Phospho-substrate profiling of Epac-dependent protein kinase C activity
Molecular and Cellular Biochemistry (2019)
-
cAMP guided his way: a life for G protein-mediated signal transduction and molecular pharmacology—tribute to Karl H. Jakobs
Naunyn-Schmiedeberg's Archives of Pharmacology (2019)
-
PACAP signaling in stress: insights from the chromaffin cell
Pflügers Archiv - European Journal of Physiology (2018)