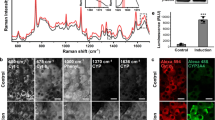
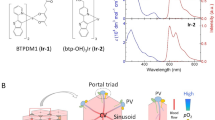
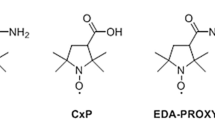
Abstract
Current drug-safety assays for hepatotoxicity rely on biomarkers with low predictive power. The production of radical species, specifically reactive oxygen species (ROS) and reactive nitrogen species (RNS), has been proposed as an early unifying event linking the bioactivation of drugs to hepatotoxicity and as a more direct and mechanistic indicator of hepatotoxic potential. Here we present a nanosensor for rapid, real-time in vivo imaging of drug-induced ROS and RNS for direct evaluation of acute hepatotoxicity. By combining fluorescence resonance energy transfer (FRET) and chemiluminescence resonance energy transfer (CRET), our semiconducting polymer–based nanosensor simultaneously and differentially detects RNS and ROS using two optically independent channels. We imaged drug-induced hepatotoxicity and its remediation longitudinally in mice after systemic challenge with acetaminophen or isoniazid. We detected dose-dependent ROS and RNS activity in the liver within minutes of drug challenge, which preceded histological changes, protein nitration and DNA double-strand-break induction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Nasr, A., Lauterio, T.J. & Davis, M.W. Unapproved drugs in the United States and the Food and Drug Administration. Adv. Ther. 28, 842–856 (2011).
Budnitz, D.S. et al. National surveillance of emergency department visits for outpatient adverse drug events. J. Am. Med. Assoc. 296, 1858–1866 (2006).
Sakatis, M.Z. et al. Preclinical strategy to reduce clinical hepatotoxicity using in vitro bioactivation data for >200 compounds. Chem. Res. Toxicol. 25, 2067–2082 (2012).
Srivastava, A. et al. Role of reactive metabolites in drug-induced hepatotoxicity. Handb. Exp. Pharmacol. 196, 165–194 (2010).
Dimasi, J.A. Risks in new drug development: approval success rates for investigational drugs. Clin. Pharmacol. Ther. 69, 297–307 (2001).
Kola, I. & Landis, J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 3, 711–715 (2004).
Tengowski, M.W. & Kotyk, J.J. Risk identification and management: MRI as a research tool in toxicology studies of new chemical entities. Prog. Drug Res. 62, 257–278 (2005).
Kramer, J.A., Sagartz, J.E. & Morris, D.L. The application of discovery toxicology and pathology towards the design of safer pharmaceutical lead candidates. Nat. Rev. Drug Discov. 6, 636–649 (2007).
Holt, M. & Ju, C. Drug-induced liver injury. Handb. Exp. Pharmacol. 196, 3–27 (2010).
Walsh, J.S. & Miwa, G.T. Bioactivation of drugs: risk and drug design. Annu. Rev. Pharmacol. Toxicol. 51, 145–167 (2011).
Antoine, D.J., Williams, D.P. & Park, B.K. Understanding the role of reactive metabolites in drug-induced hepatotoxicity: state of the science. Expert Opin. Drug Metab. Toxicol. 4, 1415–1427 (2008).
Tang, W. & Lu, A.Y. Metabolic bioactivation and drug-related adverse effects: current status and future directions from a pharmaceutical research perspective. Drug Metab. Rev. 42, 225–249 (2010).
Willmann, J.K., van Bruggen, N., Dinkelborg, L.M. & Gambhir, S.S. Molecular imaging in drug development. Nat. Rev. Drug Discov. 7, 591–607 (2008).
Pessayre, D., Mansouri, A., Berson, A. & Fromenty, B. Mitochondrial involvement in drug-induced liver injury. Handb. Exp. Pharmacol. 196, 311–365 (2010).
Russmann, S., Kullak-Ublick, G.A. & Grattagliano, I. Current concepts of mechanisms in drug-induced hepatotoxicity. Curr. Med. Chem. 16, 3041–3053 (2009).
Xiong, L., Shuhendler, A.J. & Rao, J. Self-luminescing BRET-FRET near-infrared dots for in vivo lymph-node mapping and tumour imaging. Nat. Commun. 3, 1193 (2012).
Pu, K., Shuhendler, A.J. & Rao, J. Semiconducting polymer nanoprobe for in vivo imaging of reactive oxygen and nitrogen species. Angew. Chem. Int. Edn. Engl. 52, 10325–10329 (2013).
Lee, D. et al. In vivo imaging of hydrogen peroxide with chemiluminescent nanoparticles. Nat. Mater. 6, 765–769 (2007).
Oushiki, D. et al. Development and application of a near-infrared fluorescence probe for oxidative stress based on differential reactivity of linked cyanine dyes. J. Am. Chem. Soc. 132, 2795–2801 (2010).
Hu, Y., Haynes, M.T., Wang, Y., Liu, F. & Huang, L. A highly efficient synthetic vector: nonhydrodynamic delivery of DNA to hepatocyte nuclei in vivo. ACS Nano 7, 5376–5384 (2013).
Szabo, C., Ischiropoulos, H. & Radi, R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 6, 662–680 (2007).
Smith, A.M., Mancini, M.C. & Nie, S. Bioimaging: second window for in vivo imaging. Nat. Nanotechnol. 4, 710–711 (2009).
Shuhendler, A.J. et al. Hybrid quantum dot-fatty ester stealth nanoparticles: toward clinically relevant in vivo optical imaging of deep tissue. ACS Nano 5, 1958–1966 (2011).
Pu, K. et al. Semiconducting polymer nanoparticles as photoacoustic molecular imaging probes in living mice. Nat. Nanotechnol. 10.1038/nnano.2013.302 (26 January 2014).
Herd, R.M., Dover, J.S. & Arndt, K.A. Basic laser principles. Dermatol. Clin. 15, 355–372 (1997).
Mourant, J.R., Fuselier, T., Boyer, J., Johnson, T.M. & Bigio, I.J. Predictions and measurements of scattering and absorption over broad wavelength ranges in tissue phantoms. Appl. Opt. 36, 949–957 (1997).
Jones, C.F. & Grainger, D.W. In vitro assessments of nanomaterial toxicity. Adv. Drug Deliv. Rev. 61, 438–456 (2009).
Maynard, A.D., Warheit, D.B. & Philbert, M.A. The new toxicology of sophisticated materials: nanotoxicology and beyond. Toxicol. Sci. 120 (suppl. 1), S109–S129 (2011).
Hinson, J.A., Roberts, D.W. & James, L.P. Mechanisms of acetaminophen-induced liver necrosis. Handb. Exp. Pharmacol. 196, 369–405 (2010).
Nakagawa, H. et al. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology 135, 1311–1321 (2008).
McGill, M.R. & Jaeschke, H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm. Res. 30, 2174–2187 (2013).
Gujral, J.S., Knight, T.R., Farhood, A., Bajt, M.L. & Jaeschke, H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol. Sci. 67, 322–328 (2002).
Liu, W.F. et al. Real-time in vivo detection of biomaterial-induced reactive oxygen species. Biomaterials 32, 1796–1801 (2011).
James, L.P., McCullough, S.S., Lamps, L.W. & Hinson, J.A. Effect of N-acetylcysteine on acetaminophen toxicity in mice: relationship to reactive nitrogen and cytokine formation. Toxicol. Sci. 75, 458–467 (2003).
Jackson, T.E., Lilly, P.D., Recio, L., Schlosser, P.M. & Medinsky, M.A. Inhibition of cytochrome P450 2E1 decreases, but does not eliminate, genotoxicity mediated by 1,3-butadiene. Toxicol. Sci. 55, 266–273 (2000).
Saito, C., Zwingmann, C. & Jaeschke, H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology 51, 246–254 (2010).
Saukkonen, J.J., Powell, K. & Jereb, J.A. Monitoring for tuberculosis drug hepatotoxicity: moving from opinion to evidence. Am. J. Respir. Crit. Care Med. 185, 598–599 (2012).
Metushi, I.G., Nakagawa, T. & Uetrecht, J. Direct oxidation and covalent binding of isoniazid to rodent liver and human hepatic microsomes: humans are more like mice than rats. Chem. Res. Toxicol. 25, 2567–2576 (2012).
Metushi, I.G., Cai, P., Zhu, X., Nakagawa, T. & Uetrecht, J.P. A fresh look at the mechanism of isoniazid-induced hepatotoxicity. Clin. Pharmacol. Ther. 89, 911–914 (2011).
Peet, J. et al. Efficiency enhancement in low-bandgap polymer solar cells by processing with alkane dithiols. Nat. Mater. 6, 497–500 (2007).
Sokolov, A.N., Tee, B.C., Bettinger, C.J., Tok, J.B. & Bao, Z. Chemical and engineering approaches to enable organic field-effect transistors for electronic skin applications. Acc. Chem. Res. 45, 361–371 (2012).
Wu, C. & Chiu, D.T. Highly fluorescent semiconducting polymer dots for biology and medicine. Angew. Chem. Int. Edn. Engl. 52, 3086–3109 (2013).
Guimard, N.K., Sessler, J.L. & Schmidt, C.E. Towards a biocompatible, biodegradable copolymer incorporating electroactive oligothiophene units. Macromolecules 42, 502–511 (2009).
Zhang, N., Francis, K.P., Prakash, A. & Ansaldi, D. Enhanced detection of myeloperoxidase activity in deep tissues through luminescent excitation of near-infrared nanoparticles. Nat. Med. 19, 500–505 (2013).
Park, B.K., Kitteringham, N.R., Maggs, J.L., Pirmohamed, M. & Williams, D.P. The role of metabolic activation in drug-induced hepatotoxicity. Annu. Rev. Pharmacol. Toxicol. 45, 177–202 (2005).
Cover, C. et al. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J. Pharmacol. Exp. Ther. 315, 879–887 (2005).
McGill, M.R. et al. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms, and clinical implications. Toxicol. Appl. Pharmacol. 269, 240–249 (2013).
Ghafourifar, P. & Cadenas, E. Mitochondrial nitric oxide synthase. Trends Pharmacol. Sci. 26, 190–195 (2005).
Pacher, P., Beckman, J.S. & Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 87, 315–424 (2007).
Chowdhury, A. et al. Mitochondrial oxidative stress and permeability transition in isoniazid and rifampicin induced liver injury in mice. J. Hepatol. 45, 117–126 (2006).
Li, F. et al. Human PXR modulates hepatotoxicity associated with rifampicin and isoniazid co-therapy. Nat. Med. 19, 418–420 (2013).
Lauterburg, B.H., Smith, C.V., Todd, E.L. & Mitchell, J.R. Pharmacokinetics of the toxic hydrazino metabolites formed from isoniazid in humans. J. Pharmacol. Exp. Ther. 235, 566–570 (1985).
Nathan, C. & Cunningham-Bussel, A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat. Rev. Immunol. 13, 349–361 (2013).
Li, J.O.W., Li, W., Jiang, Z.G. & Ghanbari, H.A. Oxidative stress and neurodegenerative disorders. Int. J. Mol. Sci. 14, 24438–24475 (2013).
Costa, A., Scholer-Dahirel, A. & Mechta-Grigoriou, F. The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Semin. Cancer Biol. 10.1016/j.semcancer.2013.12.007 (7 January 2014).
Acknowledgements
This work was supported by the US National Institutes of Health National Cancer Institute (NCI) grants R01CA135294, R01DK099800-06A1, R21CA138353A2, the Stanford University NCI CCNE-T grant (U54CA151459) and In Vivo Cellular and Molecular Imaging Centers grant (P50CA114747). A.J.S. acknowledges Susan G. Komen For The Cure for fellowship support. We acknowledge the use of the SCi3 Core Facility and the Neuroscience Microscopy Service Facility at Stanford University, P. Chu for her expertise in preparation of histology samples, E.J. McWalter for assistance with MatLab script, and S. Machtaler for assistance with tissue preparation and imaging by confocal microscopy.
Author information
Authors and Affiliations
Contributions
A.J.S., K.P. and J.R. conceived of the nanosensor design, and A.J.S., K.P., J.P.U. and J.R. designed the experiments. L.C. synthesized the galactose moiety. K.P. synthesized PS-g-PEG-Gal and CF-SPN, performed in vitro characterization of CF-SPN and data analysis. A.J.S. performed in vivo studies, analyzed in vivo data, and acquired histology images. A.J.S., K.P., J.P.U. and J.R. discussed the results and co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
Stanford University has filed a provisional patent application (serial number 61/841,958) to protect part of the technology described in the study.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 1215 kb)
Rights and permissions
About this article
Cite this article
Shuhendler, A., Pu, K., Cui, L. et al. Real-time imaging of oxidative and nitrosative stress in the liver of live animals for drug-toxicity testing. Nat Biotechnol 32, 373–380 (2014). https://doi.org/10.1038/nbt.2838
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.2838
This article is cited by
-
Susceptibility to radiation adverse effects in veterans with Gulf War illness and healthy civilians
Scientific Reports (2024)
-
Contributing roles of mitochondrial dysfunction and hepatocyte apoptosis in liver diseases through oxidative stress, post-translational modifications, inflammation, and intestinal barrier dysfunction
Cellular and Molecular Life Sciences (2024)
-
Antitubercular drugs: possible role of natural products acting as antituberculosis medication in overcoming drug resistance and drug-induced hepatotoxicity
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
A novel ROS-Related chemiluminescent semiconducting polymer nanoplatform for acute pancreatitis early diagnosis and severity assessment
Journal of Nanobiotechnology (2023)
-
Mitochondrial impairment but not peripheral inflammation predicts greater Gulf War illness severity
Scientific Reports (2023)