Abstract
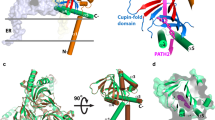
The human σ1 receptor is an enigmatic endoplasmic-reticulum-resident transmembrane protein implicated in a variety of disorders including depression, drug addiction, and neuropathic pain1. Recently, an additional connection to amyotrophic lateral sclerosis has emerged from studies of human genetics and mouse models2. Unlike many transmembrane receptors that belong to large, extensively studied families such as G-protein-coupled receptors or ligand-gated ion channels, the σ1 receptor is an evolutionary isolate with no discernible similarity to any other human protein. Despite its increasingly clear importance in human physiology and disease, the molecular architecture of the σ1 receptor and its regulation by drug-like compounds remain poorly defined. Here we report crystal structures of the human σ1 receptor in complex with two chemically divergent ligands, PD144418 and 4-IBP. The structures reveal a trimeric architecture with a single transmembrane domain in each protomer. The carboxy-terminal domain of the receptor shows an extensive flat, hydrophobic membrane-proximal surface, suggesting an intimate association with the cytosolic surface of the endoplasmic reticulum membrane in cells. This domain includes a cupin-like β-barrel with the ligand-binding site buried at its centre. This large, hydrophobic ligand-binding cavity shows remarkable plasticity in ligand recognition, binding the two ligands in similar positions despite dissimilar chemical structures. Taken together, these results reveal the overall architecture, oligomerization state, and molecular basis for ligand recognition by this important but poorly understood protein.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cobos, E. J., Entrena, J. M., Nieto, F. R., Cendán, C. M. & Del Pozo, E. Pharmacology and therapeutic potential of sigma1 receptor ligands. Curr. Neuropharmacol. 6, 344–366 (2008)
Mavlyutov, T. A., Guo, L. W., Epstein, M. L. & Ruoho, A. E. Role of the sigma-1 receptor in amyotrophic lateral sclerosis (ALS). J. Pharmacol. Sci. 127, 10–16 (2015)
Brownstein, M. J. A brief history of opiates, opioid peptides, and opioid receptors. Proc. Natl Acad. Sci. USA 90, 5391–5393 (1993)
Largent, B. L., Wikström, H., Gundlach, A. L. & Snyder, S. H. Structural determinants of sigma receptor affinity. Mol. Pharmacol. 32, 772–784 (1987)
Hanner, M. et al. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc. Natl Acad. Sci. USA 93, 8072–8077 (1996)
Su, T. P., Hayashi, T., Maurice, T., Buch, S. & Ruoho, A. E. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol. Sci. 31, 557–566 (2010)
Al-Saif, A., Al-Mohanna, F. & Bohlega, S. A mutation in sigma-1 receptor causes juvenile amyotrophic lateral sclerosis. Ann. Neurol. 70, 913–919 (2011)
Mavlyutov, T. A. et al. Lack of sigma-1 receptor exacerbates ALS progression in mice. Neuroscience 240, 129–134 (2013)
Hayashi, T. & Su, T. P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell 131, 596–610 (2007)
Wu, Z. & Bowen, W. D. Role of sigma-1 receptor C-terminal segment in inositol 1,4,5-trisphosphate receptor activation: constitutive enhancement of calcium signaling in MCF-7 tumor cells. J. Biol. Chem. 283, 28198–28215 (2008)
Kim, F. J. et al. σ 1 Receptor modulation of G-protein-coupled receptor signaling: potentiation of opioid transduction independent from receptor binding. Mol. Pharmacol. 77, 695–703 (2010)
Dussossoy, D. et al. Colocalization of sterol isomerase and sigma1 receptor at endoplasmic reticulum and nuclear envelope level. Eur. J. Biochem. 263, 377–386 (1999)
Aydar, E., Palmer, C. P., Klyachko, V. A. & Jackson, M. B. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron 34, 399–410 (2002)
Akunne, H. C. et al. The pharmacology of the novel and selective sigma ligand, PD 144418. Neuropharmacology 36, 51–62 (1997)
Lever, J. R. et al. Relationship between cerebral sigma-1 receptor occupancy and attenuation of cocaine’s motor stimulatory effects in mice by PD144418. J. Pharmacol. Exp. Ther. 351, 153–163 (2014)
John, C. S., Vilner, B. J. & Bowen, W. D. Synthesis and characterization of [125I]-N-(N-benzylpiperidin-4-yl)-4-iodobenzamide, a new σ receptor radiopharmaceutical: high-affinity binding to MCF-7 breast tumor cells. J. Med. Chem. 37, 1737–1739 (1994)
Bermack, J. E. & Debonnel, G. Distinct modulatory roles of sigma receptor subtypes on glutamatergic responses in the dorsal hippocampus. Synapse 55, 37–44 (2005)
Tagashira, H., Shinoda, Y., Shioda, N. & Fukunaga, K. Methyl pyruvate rescues mitochondrial damage caused by SIGMAR1 mutation related to amyotrophic lateral sclerosis. Biochim. Biophys. Acta 1840, 3320–3334 (2014)
Shin, E. J. et al. Dextromethorphan attenuates trimethyltin-induced neurotoxicity via σ1 receptor activation in rats. Neurochem. Int. 50, 791–799 (2007)
Roth, B. L., Lopez, E., Patel, S. & Kroeze, W. K. The multiplicity of serotonin receptors: uselessly diverse molecules or an embarrassment of riches? Neuroscientist 6, 252–262 (2000)
Seth, P. et al. Expression pattern of the type 1 sigma receptor in the brain and identity of critical anionic amino acid residues in the ligand-binding domain of the receptor. Biochim. Biophys. Acta 1540, 59–67 (2001)
Yamamoto, H. et al. Amino acid residues in the transmembrane domain of the type 1 sigma receptor critical for ligand binding. FEBS Lett. 445, 19–22 (1999)
Kovács, K. J. & Larson, A. A. Up-regulation of [3H]DTG but not [3H](+)-pentazocine labeled sigma sites in mouse spinal cord by chronic morphine treatment. Eur. J. Pharmacol. 350, 47–52 (1998)
Martin, W. R., Eades, C. G., Thompson, J. A., Huppler, R. E. & Gilbert, P. E. The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 197, 517–532 (1976)
Nguyen, L. et al. Role of sigma-1 receptors in neurodegenerative diseases. J. Pharmacol. Sci. 127, 17–29 (2015)
Mei, J. & Pasternak, G. W. σ1 Receptor modulation of opioid analgesia in the mouse. J. Pharmacol. Exp. Ther. 300, 1070–1074 (2002)
Maurice, T. & Su, T. P. The pharmacology of sigma-1 receptors. Pharmacol. Ther. 124, 195–206 (2009)
Gromek, K. A. et al. The oligomeric states of the purified sigma-1 receptor are stabilized by ligands. J. Biol. Chem. 289, 20333–20344 (2014)
Mishra, A. K. et al. The sigma-1 receptors are present in monomeric and oligomeric forms in living cells in the presence and absence of ligands. Biochem. J. 466, 263–271 (2015)
Lomize, M. A., Pogozheva, I. D., Joo, H., Mosberg, H. I. & Lomize, A. L. OPM database and PPM web server: resources for positioning of proteins in membranes. Nucleic Acids Res. 40, D370–D376 (2012)
Caffrey, M. & Cherezov, V. Crystallizing membrane proteins using lipidic mesophases. Nature Protocols 4, 706–731 (2009)
Rasmussen, S. G. et al. Structure of a nanobody-stabilized active state of the β2 adrenoceptor. Nature 469, 175–180 (2011)
Bricogne, G., Vonrhein, C., Flensburg, C., Schiltz, M. & Paciorek, W. Generation, representation and flow of phase information in structure determination: recent developments in and around SHARP 2.0. Acta Crystallogr. D 59, 2023–2030 (2003)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D 68, 352–367 (2012)
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010)
Schrodinger, LLC. The PyMOL Molecular Graphics System, v.1.3r1 (2010)
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004)
Morin, A. et al. Collaboration gets the most out of software. eLife 2, e01456 (2013)
Ashkenazy, H., Erez, E., Martz, E., Pupko, T. & Ben-Tal, N. ConSurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res. 38, W529–W533 (2010)
Holm, L. & Rosenström, P. Dali server: conservation mapping in 3D. Nucleic Acids Res. 38, W545–W549 (2010)
Strop, P. & Brunger, A. T. Refractive index-based determination of detergent concentration and its application to the study of membrane proteins. Protein Sci. 14, 2207–2211 (2005)
Reisinger, V. & Eichacker, L. A. Analysis of membrane protein complexes by blue native PAGE. Proteomics 6 (Suppl. 2), 6–15 (2006)
Acknowledgements
We thank beamline staff at the Advanced Photon Source for their technical assistance and support. We thank S. Blacklow and B. Zimmerman for discussions and input throughout the project, and K. Arnett for assistance with SEC–MALS experiments. Financial support for this work was provided by departmental startup funds. S.Z. is supported by a Merck Postdoctoral Fellowship in Biological Chemistry and Molecular Pharmacology, and H.R.S. is supported by the National Institutes of Health Cellular and Developmental Biology training grant at Harvard Medical School (T32GM007226).
Author information
Authors and Affiliations
Contributions
Receptor purification and crystallization experiments were conducted by H.R.S., E.G., and A.C.K. X-ray data collection was performed by H.R.S., A.M., A.K., and A.C.K. Data processing, phase calculation, and structure refinement were performed jointly by H.R.S., S.Z., and A.C.K. SEC–MALS experiments were performed by A.C.K., radioligand binding by H.R.S., and native-PAGE by S.Z. Overall project design, molecular cloning, and pilot studies were conducted by A.M. and A.C.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Assessment of σ1 functional properties and biochemical quality.
a, Saturation binding curve to measure Kd for 3H (+)-pentazocine, with points shown as mean ± s.e.m. b, Competition binding measurement of affinities for the two co-crystallized ligands with points shown as mean ± s.e.m. c, Summary of binding affinities with 95% confidence intervals for Kd/Ki values. d, Analysis of receptor purity by SDS–PAGE. e, Analytical size exclusion of purified σ1 receptor in LMNG/CHS detergent buffer on a Superdex 200 column.
Extended Data Figure 2 Representative electron density.
a, Composite omit 2Fo − Fc electron density contoured at 1.0σ for σ1 receptor bound to PD144418, showing a loop from Val73 to Glu78 as well as surrounding residues. b, The same map over a loop from His116 to Ser125. c, The equivalent map to that in a, calculated for σ1 receptor bound to 4-IBP. d, The equivalent map to that in b, calculated for σ1 receptor bound to 4-IBP.
Extended Data Figure 3 Lattice contacts.
a, Lattice packing of the σ1 receptor viewed parallel to the membrane plane. b, c A view normal to the membrane and another parallel view, respectively. d, A single σ1 trimer is shown, with lattice contact residues highlighted in magenta sticks. Lattice contacts are formed primarily through interactions of the relatively poorly conserved transmembrane helices.
Extended Data Figure 4 Hydrophobicity analysis.
a, The structure of σ1 receptor shows a hydrophilic (blue) surface on the cytosolic face (left), while transmembrane domains and the membrane-facing surface of the receptor trimer are hydrophobic (orange; right panels). Hydrophobicity analysis was conducted using UCSF Chimera.
Extended Data Figure 5 Sequence conservation.
The results of an alignment of 277 sigma receptor sequences from vertebrates with Homo sapiens, Mus musculus, Danio rerio, and Xenopus laevis displayed. Residues with 98%, 80%, and 60% similarity are shown in black, grey, and light grey respectively. Secondary structure elements are shown above the alignment on the basis of the human σ1 receptor crystal structure. Open black circles mark residues within 4 Å of the ligand-binding site, solid black circles below the alignment denote residues located in the trimerization interface, and a red circle marks the site of the E102Q mutation associated with amyotrophic lateral sclerosis.
Extended Data Figure 6 Trimerization interface.
a, b, Two views of the trimerization interface are shown, coloured by sequence conservation. Residues highlighted in yellow are more than 80% conserved among a selection of 300 σ1 receptor homologues, and residues in orange surface are more than 98% conserved. c, d, Close-up views of the interface, showing the extensive hydrophobic and polar contacts at the oligomerization interface.
Extended Data Figure 7 Omit maps of PD144418 and 4-IBP.
a, An Fo − Fc omit map contoured at 1σ showing the electron density (purple) of PD144418 (yellow). b, An equivalent map showing the electron density (purple) of 4-IBP (orange).
Extended Data Figure 8 Oligomerization state.
a, Analysis of receptor oligomerization by SEC–MALS in the presence of the classical antagonist NE-100 or (b) the classical agonist SKF-10,047. The peak is 38% detergent and 62% protein by mass. The total mass of each component varies throughout the peak, indicating a mix of oligomeric species. c, Analysis of oligomerization state by blue native PAGE (left) and a higher-resolution detergent-supplemented tris-glycine native PAGE gel (right), showing a similar polydisperse profile. Discrete oligomers are marked with red dots, corresponding to possible trimers, hexamers, and higher-order species. d, In a mixed micelle of lauryl maltose neopentyl glycol and cholesterol hemisuccinate, modest differences in SEC profile are observed between agonist- and antagonist-treated receptor.
Rights and permissions
About this article
Cite this article
Schmidt, H., Zheng, S., Gurpinar, E. et al. Crystal structure of the human σ1 receptor. Nature 532, 527–530 (2016). https://doi.org/10.1038/nature17391
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature17391
This article is cited by
-
Benzomorphan and non-benzomorphan agonists differentially alter sigma-1 receptor quaternary structure, as does types of cellular stress
Cellular and Molecular Life Sciences (2024)
-
Nitroalkanes as thioacyl equivalents to access thioamides and thiopeptides
Nature Communications (2023)
-
An open-like conformation of the sigma-1 receptor reveals its ligand entry pathway
Nature Communications (2022)
-
Sigma non-opioid receptor 1 is a potential therapeutic target for long QT syndrome
Nature Cardiovascular Research (2022)
-
Discovery of novel, selective, functionalized 5-(2-(5-arylhexahydropyrrolo[3,4-c]pyrrol-2(1H)-yl)ethyl)-γ-butyrolactone sigma-2 ligands
Medicinal Chemistry Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.