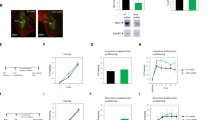
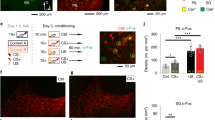
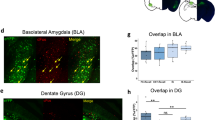
Abstract
The amygdala plays a critical role in the mediation of emotional responses, particularly fear, in both humans and animals1,2,3,4. Fear conditioning, a conditioned learning paradigm, has served as a model for emotional learning in animals, and the neuroanatomical circuitry underlying the auditory fear-conditioning paradigm is well characterized5. Synaptic transmission in the medial geniculate nucleus (MGN) to lateral nucleus of the amygdala (LA) pathway, a key segment of the auditory fear conditioning circuit, is mediated largely through N-methyl-D-aspartate (NMDA) and non-NMDA (such as α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)) glutamate receptors6; the potential for neural plasticity in this pathway is suggested by its capacity to support long-term potentiation (LTP)7,8. Here we report a long-lasting increase in the synaptic efficacy of the MGN–LA pathway attributable to fear-conditioning itself, rather than an electrically induced model of learning. Fear-conditioned animals show a presynaptic facilitation of AMPA-receptor-mediated transmission, directly measured in vitro with whole-cell recordings in lateral amygdala neurons. These findings represent one of the first in vitro measures of synaptic plasticity resulting from emotional learning by whole animals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Scott, S. K. et al. Impaired auditory recognition of fear and anger following bilateral amygdala lesions. Nature 385, 254–257 (1997).
Aldolphs, R., Tranel, D., Damasio, H. & Damasio, A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372, 669–672 (1994).
Maren, S. Synaptic transmission and plasticity in the amygdala: An emerging physiology of fear conditioning circuits. Mol. Neurobiol. 13, 1–22 (1996).
Davis, M., Rainnie, D. & Cassell, M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 17, 208–214 (1994).
LeDoux, J. E. Emotion: clues from the brain. Annu. Rev. Psychol 46, 209–235 (1995).
Li, X. F., Phillips, R. & LeDoux, J. E. NMDA and non-NMDA receptors contribute to synaptic transmission between the medial geniculate body and the lateral nucleus of the amygdala. Exp. Brain Res. 105, 87–100 (1995).
Clugnet, M. C. & LeDoux, J. E. Synaptic plasticity in fear conditioning circuits: induction of LTP in the lateral nucleus of the amygdala by stimulation of the medial geniculate body. J. Neurosci. 10, 2818–2824 (1990).
Rogan, M. T. & LeDoux, J. E. LTP is accompanied by commensurate enhancement of auditory-evoked responses in a fear conditioning circuit. Neuron 15, 127–136 (1995).
Davis, M., Falls, W. A., Campeau, S. & Munsoo, K. Fear-potentiated startle: a neural and pharmacological analysis. Behav. Brain Res. 58, 175–198 (1993).
Kim, M., Campeau, S., Falls, W. A. & Davis, M. Infusion of the non-NMDA receptor antagonist CNQX into the amygdala blocks the expression of fear-potentiated startle. Behav. Neural Biol. 59, 5–8 (1993).
Miserendino, M. J. D., Sananes, C. B., Melia, K. R. & Davis, M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature 345, 716–718 (1990).
LeDoux, J. E., Ruggiero, D. A. & Reis, D. J. Projections from anatomically defined regions of the medial geniculate body in the rat. J. Comp. Neurol. 242, 182–213 (1985).
Parsons, C. G., Gruner, R. & Rozental, J. Comparative patch clamp studies on the kinetics and selectivity of glutamate receptor antagonism by 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline (NBQX) and 1-(4-aminophenyl)-4-methyl-7,8-methyl-endoxyl-5H-2,3-benzodiazepine (GYKI 52466). Neuropharmacology 33, 589–604 (1994).
Muller, D., Joly, M. & Lynch, G. Differential contributions of quisqualate versus NMDA receptors to the induction and expression of long-term potentiation. Science 242, 1694–1697 (1988).
Kauer, J., Malenka, R. & Nicoll, R. Apersistent postsynaptic modification mediates long-term potentiation in the hippocampus. Neuron 1, 911–917 (1988).
Kullman, D. Amplitude fluctuations of dual-component EPSCs in hippocampal pyramidal cells: Implications for long-term potentiation. Neuron 12, 1111–1120 (1994).
Katz, B. & Miledi, R. The role of calcium in neuromuscular facilitation. J. Physiol. 195, 481–492 (1968).
Creager, R., Dunwiddie, T. & Lynch, G. Paired-pulse and frequency facilitation in the CA1 region of the in vitro rat hippocampus. J. Physiol. 299, 409–424 (1980).
Manabe, T., Wyllie, D. J. A., Perkel, D. J. & Nicoll, R. A. Modulation of synaptic transmission and long-term potentiation: effects on paired-pulse facilitation and EPSC variance in the CA1 region of the hippocampus. J. Neurophys. 70, 1451–1459 (1993).
Kuhnt, U. & Voronin, L. L. Interaction between paired-pulse facilitation and long-term potentiation in area CA1 of guinea-pig hippocampal slices: application of quantal analysis. Neuroscience 62, 391–397 (1994).
Shulz, P., Cook, E. & Johnston, D. Changes in paired-pulse facilitation suggest presynaptic involvement in long-term potentiation. J. Neurosci. 14, 5325–5337 (1994).
Clark, K. A., Randall, A. D. & Collinridge, G. L. Acomparison of paired-pulse facilitation of AMPA and NMDA receptor-mediated excitatory postsynaptic currents in the hippocampus. Exp. Brain Res. 101, 272–278 (1994).
Muller, D. & Lynch, G. Synaptic modulation of N-methyl-D-aspartate receptor-mediated responses in hippocampus. Synapse 5, 94–103 (1990).
Lin, J. & Faber, D. Synaptic transmission mediated by single club endings on the goldfish Mauthner cell. II. Plasticity of excitatory ostsynaptic potentials. J. Neurosci. 8, 1313–1325 (1988).
McKernan, M. & Shinnick-Gallagher, P. Paired-pulse facilitation (PPF) in an internal capsule-lateral amygdala pathway. Soc. Neurosci. Abstr. 22, 1536 (1996).
Neugebauer, V., Keele, N. B. & Shinnick-Gallagher, P. Epileptogenesis in vivo enhances the sensitivity of inhibitory presynaptic metabotropic glutamate receptors in basolateral amygdala neurons in vitro. J. Neurosci. 17, 983–995 (1997).
Blanton, M. G., Lo Turco, J. J. & Kriegstein, A. R. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J. Neurosci. Meth. 30, 203–210 (1989).
Andreasen, M. & Hablitz, J. Paired-pulse facilitation in the dentate gyrus: A patch-clamp study in the rat hippocampus in vitro. J. Neurophysiol. 72, 326–336 (1994).
Cassella, J. V. & Davis, M. The design and calibration of a startle measurement system. Physiol. and Behav. 36, 377–383 (1986).
Acknowledgements
We thank J. E. Blankenship, B. Christensen, J. Gallagher, K. Johnson, M. Thomas and V. Neugebauer for critically reading the manuscript, and K. Cunningham for statistical assistance. This work was supported by the John Sealy Memorial Endowment Fund for Biomedical Research (P.S.G.), an NIMH National Research Service Award (M.G.M.) and a PhRMA Medical Student Research Fellowship in Pharm.-Clinical Pharmacology (M.G.M.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McKernan, M., Shinnick-Gallagher, P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature 390, 607–611 (1997). https://doi.org/10.1038/37605
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/37605
This article is cited by
-
Closed-loop brain stimulation augments fear extinction in male rats
Nature Communications (2023)
-
Thalamus sends information about arousal but not valence to the amygdala
Psychopharmacology (2023)
-
N-acetylcysteine facilitates extinction of cued fear memory in rats via reestablishing basolateral amygdala glutathione homeostasis
Acta Pharmacologica Sinica (2022)
-
Prolonged contextual fear memory in AMPA receptor palmitoylation-deficient mice
Neuropsychopharmacology (2022)
-
Neurotensin orchestrates valence assignment in the amygdala
Nature (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.