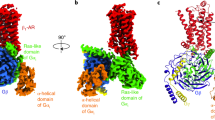
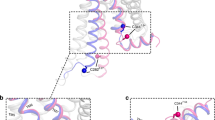
Abstract
REGULATORY GTP-binding proteins (G proteins) are membrane-attached heterotrimers (α, β, γ) that mediate cellular responses to a wide variety of extracellular stimuli1,2. They undergo a cycle of guanine-nucleotide exchange and GTP hydrolysis, during which they dissociate into αsubunit and βγ complex1. The roles of G-protein αsubunits in these processes and for the specificity of signal transduction are largely established; the α- and γ-subunits are essential for receptor-induced G-protein activation and seem to be less diverse and less specific. Although the complementary DNAs for several β-subunits have been cloned2,5–8, isolated sub-units have only been studied as βγ complexes3,9–12. Functional differences have been ascribed to the γ-subunit on the basis of extensive sequence similarity among β-subunits and apparent heterogeneity in γ-subunit sequences13,14.βγ complexes can interact directly or indirectly with different effectors10,11,15–20. They seem to be interchangeable in their interaction with pertussis toxinsensitive α-subunits3, so we tested this by microinjecting antisense oligonucleotides into nuclei of a rat pituitary cell line to suppress the synthesis of individual β-subunits selectively. Here we show that two out of four subtypes of β-subunits tested (β1 and β3) are selectively involved in the signal transduction cascades from muscarinic M4 (ref. 4) and somatostatin receptors, respectively, to voltage-dependent Ca2+ channels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Birnbaumer, L., Abramowitz, J. & Brown, A. M. Biochim. biophys. Acta 1031, 163–224 (1990).
Simon, M. I., Strathmann, M. P. & Gautam, N. Science 252, 802–808 (1991).
Hekman, M. et al. Eur. J. Biochem. 169, 431–439 (1987).
Pinkas-Kramarski, R., Edelman, R. & Stein, R. Neurosci. Lett. 108, 335–340 (1990).
Codina, J., Stengel, D., Woo, S. L. C. & Birnbaumer, L. FEBS Lett. 207, 187–192 (1986).
Gao, B., Gilman, A. G. & Robishaw, J. D. Proc. natn. Acad. Sci. U.S.A. 84, 6122–6125 (1987).
Levine, M. A., Smallwood, P. M., Moen, P. T. Jr, Helman, L. J. & Ahn, T. G. Proc. natn. Acad. Sci. U.S.A. 87, 2329–2333 (1990).
Von Weizsäcker, E., Strathmann, M. P. & Simon, M. I. Biochem. biophys. res. Commun. 183, 350–356 (1992).
Simonds, W. F., Butrynski, J. E., Gautam, N., Unson, C. G. & Spiegel, A. M. J. biol. Chem. 266, 5363–5366 (1991).
Cerione, R. A. et al. Biochemistry 26, 1485–1491 (1987).
Fawzi, A. B. et al. J. biol. Chem. 266, 12194–12200 (1991).
Kanaho, Y. et al. J. biol. Chem. 259, 7378–7381 (1984).
Tamir, H., Fawzi, A. B., Tamir, A., Evans, T. & Northup, J. K. Biochemistry 30, 3929–3936 (1991).
Fisher, K. J. & Aronson, N. N. Jr Molec. cell. Biology 12, 1585–1591 (1992).
Katada, T., Kusakabe, K., Oinuma, M. & Ui, M. J. biol. Chem. 262, 11897–11900 (1987).
Jelsema, C. L. & Axelrod, J. Proc. natn. Acad. Sci. U.S.A. 84, 3623–3627 (1987).
Logothetis, D. E., Kurachi, Y., Galper, J., Neer, E. J. & Clapham, D. E. Nature 325, 321–326 (1987).
Okabe, K. et al. J. biol. Chem. 265, 12854–12858 (1990).
Kim, D. et al. Nature 337, 557–560 (1989).
Boyer, J. L. J. biol. Chem. 264, 13917–13922 (1989).
Kleuss, C. et al. Nature 353, 43–48 (1991).
Law, S. F., Manning, D. & Reisine, T. J. biol. Chem. 266, 17885–17897 (1991).
Church, G. M. & Gilbert, W. Proc. natn. Acad. Sci. U.S.A. 81, 1991–1995 (1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kleuss, C., Scherübl, H., Hescheler, J. et al. Different β-subunits determine G-protein interaction with transmembrane receptors. Nature 358, 424–426 (1992). https://doi.org/10.1038/358424a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/358424a0
This article is cited by
-
Oscillatory calcium release and sustained store-operated oscillatory calcium signaling prevents differentiation of human oligodendrocyte progenitor cells
Scientific Reports (2022)
-
The in vivo specificity of synaptic Gβ and Gγ subunits to the α2a adrenergic receptor at CNS synapses
Scientific Reports (2019)
-
Association of Genetic Variants in GNβ3 with Functional Dyspepsia: A Meta-Analysis
Digestive Diseases and Sciences (2014)
-
Homozygous 825T Allele of the GNB3 Protein Influences the Susceptibility of Japanese to Dyspepsia
Digestive Diseases and Sciences (2008)
-
Heterotrimeric G‐proteins: a short history
British Journal of Pharmacology (2006)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.