Abstract
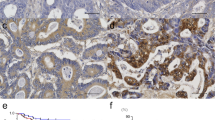
Bile acids have been implicated in the development of colorectal cancers. We investigated the ex-pressionof the transcription factor regulated by bile acids, farnesoid X receptor (FXR), as well asother components of this pathway in human colorectal tumors and cell lines. The most significantchanges were a decrease in FXR mRNA levels in adenomas (5-fold average) and carcinomas (10fold average) and an increase in peroxisome proliferator activated receptor-γ (2-fold average). FXRwas not expressed in undifferentiated colon adenocarcinoma SW480 cells and metastasis derivedSW620 cells. In Caco-2 and HT-29 cells, the level of FXR expression increased with the degree ofdifferentiation. Intestinal bile acid binding protein was activated by chenodeoxycholic acid and thesynthetic FXR agonist GW4064 in Caco-2 and HT-29 but not in SW cells unless FXR was trans-fected.The down-regulation of the nuclear receptor FXR in colon cancer might be of clinical andpharmacological importance.
Similar content being viewed by others
REFERENCES
Kay RM: Effects of diet on the fecal excretion and bacterial modification of acidic and neutral steroids, and implications for colon carcinogenesis. Cancer Res 41:3774–3777, 1981
Breuer N, Goebell H: The role of bile acids in colonic carcinogenesis. Klin Wochenschr 63:97–105, 1985
Nagengast FM, Grubben MJ, van Munster IP: Role of bile acids in colorectal carcinogenesis. Eur J Cancer 31A:1067–1070, 1995
Willett WC, Stampfer MJ, Colditz GA, Rosner BA, Speizer FE: Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. N Engl J Med 323:1664–1672, 1990
Narisawa T, Reddy BS, Weisburger JH: Effect of bile acids and dietary fat on large bowel carcinogenesis in animal models. Gastroenterol Jpn 13:206–212, 1978
Giovannucci E, Colditz GA, Stampfer MJ: A meta-analysis of cholecystectomy and risk of colorectal cancer. Gastroenterology 105:130–141, 1993
Todoroki I, Friedman GD, Slattery ML, Potter JD, Samowitz W: Cholecystectomy and the risk of colon cancer. Am J Gastroenterol 94:41–46, 1999
Lagergren J, Ye W, Ekbom A: Intestinal cancer after cholecystectomy: is bile involved in carcinogenesis? Gastroenterology 121:542–547, 2001
de Kok TM, van Faassen A, Glinghammar B, Pachen DM, Eng M, Rafter JJ, Baeten CG, et al.: Bile acid concentrations, cytotoxicity, and pH of fecal water from patients with colorectal adenomas. Dig Dis Sci 44:2218–2225, 1999
Imray CH, Radley S, Davis A, Barker G, Hendrickse CW, Donovan IA, Lawson AM, et al.: Faecal unconjugated bile acids in patients with colorectal cancer or polyps. Gut 33:1239–1245, 1992
Bayerdorffer E, Mannes GA, Ochsenkuhn T, Dirschedl P, Wiebecke B, Paumgartner G: Unconjugated secondary bile acids in the serum of patients with colorectal adenomas. Gut 36:268–273, 1995
Hori T, Matsumoto K, Sakaitani Y, Sato M, Morotomi M: Effect of dietary deoxycholic acid and cholesterol on fecal steroid concentration and its impact on the colonic crypt cell proliferation in azoxymethane-treated rats. Cancer Lett 124:79–84, 1998
Kanamoto R, Azuma N, Suda H, Seki T, Tsuchihashi Y, Iwami K: Elimination of Na+-dependent bile acid transporter from small intestine by ileum resection increase colonic tumorigenesis in the rat fed deoxycholic acid. Cancer Lett 145:115–120, 1999
Wang W, Xue S, Ingles SA, Chen Q, Diep AT, Frankl HD, Stolz A, et al.: An association between genetic polymorphisms in the ileal sodium-dependent bile acid transporter gene and the risk of colorectal adenomas. Cancer Epidemiol Biomarkers Prev 10:931–936, 2001
Davidson LA, Lupton JR, Jiang YH, Chang WC, Aukema HM, Chapkin RS: Dietary fat and fiber alter rat colonic protein kinase C isozyme expression. J Nutr 125:49–56, 1995
Schlottman K, Wachs FP, Krieg RC, Kullmann F, Scholmerich J, Rogler G: Characterization of bile salt-induced apoptosis in colon cancer cell lines. Cancer Res 60:4270–4276, 2000
Milovic V, Teller IC, Faust D, Caspary WF, Stein J: Effects of de-oxycholate on human colon cancer cells: apoptosis or proliferation. Eur J Clin Invest 32:29–34, 2002
Pool-Zobel BL, Leucht U: Induction of DNA damage by risk factors of colon cancer in human colon cells derived from biopsies. Mutat Res 375:105–115, 1997
Hirano F, Tanada H, Makino Y, Okamoto K, Hiramoto M, Handa H, Makino I: Induction of the transcription factor AP-1 in cultured human colon adenocarcinoma cells following exposure to bile acids. Carcinogenesis 17:427–433, 1996
Repa JJ, Mangelsdorf DJ: The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu Rev Cell Dev Biol 16:459–481, 2000
Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ: An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature 383:728–731, 1990
Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, et al.: Identification of a nuclear receptor for bile acids. Science 284:1362–1365, 1999
Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, et al.: Bile acids: Natural ligands for an orphan nuclear receptor. Science 284:1365–1368, 1999
Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ: Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell 6:507–515, 2000
Repa JJ, Turely SD, Lobaccaro JA, Medina J, Li L, Lustig K, Shan B, et al.: Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 289:1524–1529, 2000
DuBois RN, Gupta R, Brockman J, Reddy BS, Krakow SL, Lazar MA: The nuclear eicosanoid receptor, PPARgamma, is aberrantly expressed in colonic cancers. Carcinogenesis 19:49–53, 1998
Koeffler HP: Peroxisome proliferator-activated receptor gamma and cancers. Clin Cancer Res 9:1–9, 2003
Fabricant M, Broitman SA: Evidence for deficiency of low density lipoprotein receptor on human colonic carcinoma cell lines. Cancer Res 50:632–636, 1990
Maloney PR, Parks DJ, Haffner CD, Fivush AM, Chandra G, Plunket KD,Creech KL, et al.: Identification of a chemical tool for the orphan nuclear receptor FXR. J Med Chem 43:2971–2974, 2000
Dufour JF, Luthi M, Forestier M, Magnino F: Expression of inositol 1,4,5-trisphosphate receptor isoforms in rat cirrhosis. Hepatology 30:1018–1026, 1999
Grober J, Zaghini I, Fujii H, Jones SA, Kliewer SA, Willson TM, Ono T, et al.: Identification of a bile acid-responsive element in the human ileal bile acid-binding protein gene. Involvement of the farnesoid X receptor/9-cis-retinoic acid receptor heterodimer. J Biol Chem 274:29749–29754, 1999
Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ: Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102:731–744, 2000
Zaghini T, Landrier JF, Grober J, Krief S, Jones SA, Monnot MC, Lefrere I, et al.: Sterol regulatory element-binding protein-1c is responsible for cholesterol regulation of ileal bile acid-binding protein gene in vivo. Possible involvement of liver-X-receptor. J Biol Chem 277:1324–1331, 2002
Rousset M, Laburthe M, Pinto M, Chevalier G, Rouyer-Fessard C, Dussaulx E, Trugnan G, et al.: Enterocytic differentiation and glucose utilization in the human colon tumor cell line Caco-2: Modulation by forskolin. J Cell Physiol 123:377–385, 1985
Brown R, Strathdee G: Epigenomics and epigenetic therapy of cancer. Trends Mol Med 8:S43–S48, 2002
Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP: CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA 96:8681–8686, 1999
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
De Gottardi, A., Touri, F., Maurer, C.A. et al. The Bile Acid Nuclear Receptor FXR and the Bile Acid Binding Protein IBABP Are Differently Expressed in Colon Cancer. Dig Dis Sci 49, 982–989 (2004). https://doi.org/10.1023/B:DDAS.0000034558.78747.98
Issue Date:
DOI: https://doi.org/10.1023/B:DDAS.0000034558.78747.98