Abstract
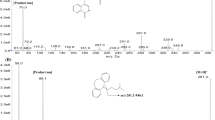
Depsipeptide, a cyclic peptide (FR), isolated from Chrombacterium violaceum strain WB968 by Fujisawa Company during a screening program for anti-oncogene agents, possesses potent antitumor activity against human tumor cell lines and xenografts. This compound has been selected for preclinical and early clinical development by the National Cancer Institute. The pharmacokinetics and oral bioavailability of this depsipeptide in the rat were investigated in the present study. A sensitive and specific electrospray LC-tandem mass spectrometry method was first developed and validated for the analysis of this depsipeptide in plasma using t-boc-α-d-glutamic acid benzyl ester as the internal standard. The routine sensitivity limit was 1 or 10 ng/ml using 1.0 or 0.1 ml of plasma sample. The within-run CV values were 11.8, 17.9, 11.0, and 5.0% at 1, 10, 100, and 500 ng/ml levels, respectively, with corresponding accuracy of 94.4, 109, 95, and 97% (all n=6). A formulation based on ethanol, normal saline and PEG400 was then developed and Fischer rats were given this formulated drug separately by intravenous and oral route. Plasma drug concentrations were measured by this method and pharmacokinetics were analyzed by the standard techniques. Plasma concentration-time profiles were found to follow a biexponential decline with a mean terminal t1/2 of 97 min and mean total clearance (CLt) of 425.3 ml/min/kg following iv dosing at 10 mg/kg. Following oral dosing at 50 mg/kg, the peptide was absorbed but produced erratic drug levels also with a bioavailability of 15.6%. Thus, active plasma concentrations can be produced up to 3 hrs in the rat following a single dose at 10 mg/kg and the peptide represents one of the very few orally absorbed peptides reported.
Similar content being viewed by others
References
Ueda H, Nakajim H, Hori Y, Fujita T, Nishimura M, Goto T, Okuhara M: FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum No. 968. I. Taxonomy, fermentation, isolation, physico-chemical and biological properties, and antitumor activity. J Antibiotics 47: 301–310, 1994.
Ueda H, Nakajima H, Hori Y, Goto T, Okuhara M: Action of FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum No. 968, on Ha-ras transformed NIH3T3 cells. Biosci Biotech Biochem 58(9): 1579–1583, 1994.
Ueda H, Manda T, Matsumoto S, Mukumoto S, Nishigaki F, Shimomura K: FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum No. 968. III. Antitumor activities on experimental tumors in mice. J Antibiotics 47: 315–323, 1994.
Page JG, Rodman LE, Heath JE, Tomaszewski JE, Smith AC: Effect of infusion rat on the toxicity of depsipeptide (NSC-630176) in beagle dogs. Proc Am Assoc Cancer Res 36: 368, 1995.
Hong PS, Chan KK: Identification and quantitation of alcophosphamide, a metabolite of cyclophosphamide, in the rat using chemical ionization mass spectrome try. Biomed Mass Spectrom 14: 167–172, 1987.
Gibaldi M, Perrier D: Pharmacokinetics. 2nd ed, Marcel-Dekker, New York, 1982.
Fouda H, Nocerini M, Schneider R, Gedutis C: Quantitative analysis by high performance liquid chromatography atmospheric pressure chemical ionization mass spectrometry: the determination of the renin inhibitor CP-80, 794 in human serum. J Am Soc Mass Spectrom 2: 164–167, 1991.
Gilbert JD, Hand EL, Yuan AS, Olah TV, Covey TR: Determination of L-365, 260, a new cholecytokinin receptor (CCK-B) antagonist, in plasma by liquid chromatography/atmospheric pressure chemical ionization mass spectrometry. Biol Mass Spectrom 21: 63–68, 1992.
Kaye B, Clark MWH, Cussans NJ, Macrae PV, Stopher DA: The sensitive determination of abanoquid in blood by high-performance liquid chromatograph/atmospheric pressure ionization mass spectrometry. Biol Mass Spectrom 21: 585–589, 1992.
Lee HJ: Biopharmaceutical properties and pharmacokinetics of peptide and protein drugs. In Taylor MD and Amidon GL (eds) Peptide-Based Drug Design. Am Chem Soc, Washington, DC, 1995, pp 69–147.
Lee VHL, Dodda-Kashi S, Grass GM, Rubas W: Oral route of peptide and protein drug delivery. In Lee VHL (ed) Peptide and Protein Drug Delivery. Marcel-Dekker, New York, 1991, pp 691–718.
Hu M and Amidon G: Passive and carrier-mediated intestinal absorption components of captopril. J Pharm Sci 77(12): 1007–1011, 1988.
Friedman DI, Amidon GL: Passive and carrier-mediated intestinal absorption components of two antiotensin converting enzyme (ACE) inhibitor produgs in rats: enalapril and fosinopril. Pharm Res 6(12): 1043–1047, 1989.
Yee S and Amidon GL: Oral absorption of antiotensin-converting enzyme inhibitors and peptide prodrugs. In Tayor MD and Amidon GL (eds) Peptide-Based Drug Design. Am Chem Soc, Washington, DC, 1995, pp 135–136.
Ptachcinski RJ, Venkataramanan R, Rosenthal JT, Burckart GJ, Taylor RJ, Hakala TR: The effect of food on cyclosporin absorption. Transplantation 40: 174–176, 1985.
Ptachcinski RJ, Venkataramanan R, Burckart GJ: Clinical pharmacokinetics of cyclosporin. Clin Pharmacokin 11(2): 107–132, 1986.
Murata K, Noda K, Kohno K, Samejima M: Pharmacokinetic analysis of concentration data of drugs with irregular absorption profiles using multi-fraction absorption models. J Pharm Sci 76: 109–113.
Suttle AB, Pollack GM, Brouwer KLR (1992): Use of pharmacokinetic model incorporating discontinuous gastrointestinal absorption to examine the occurrence of double peaks in oral concentration-time profiles. Pharm Res 9: 350–356, 1987.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chan, K.K., Bakhtiar, R. & Jiang, C. Depsipeptide (FR901228, NSC-630176) pharmacokinetics in the rat by LC/MS/MS. Invest New Drugs 15, 195–206 (1997). https://doi.org/10.1023/A:1005847703624
Issue Date:
DOI: https://doi.org/10.1023/A:1005847703624