Abstract
Background
Hyperphosphatemia is a common complication in dialysis patients that can be treated by oral phosphate binders. We investigated the efficacy and safety of oral ferric citrate as a phosphate binder for Taiwanese patients with end stage renal disease and with hyperphosphatemia who were undergoing hemodialysis.
Methods
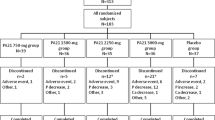
This was a prospective, double-blind, placebo-controlled, randomized trial carried out in 5 hospitals in Taiwan. Ferric citrate (4 or 6 g/day) or placebo was administered for 56 days. Serum calcium, phosphorous levels, calcium × phosphorus product, serum ferritin level, transferrin saturation, and adverse events were recorded.
Results
A total of 166 patients completed the trial. The placebo group had relatively constant serum data. Serum phosphorus declined significantly in the 6 g/day group (p < 0.05 for 4 and 8 weeks) and the 4 g/day group (p < 0.05 for 4 and 8 weeks). There were similar changes in the calcium × phosphorus product. The serum ferritin level increased significantly in the 6 g/day group (p < 0.05) and the 4 g/day group (p < 0.05). There were similar changes in transferrin saturation. Most adverse events were mild to moderate and were comparable among the three groups.
Conclusions
A 56-day treatment with ferric citrate effectively controlled hyperphosphatemia and was well tolerated in maintenance hemodialysis patients. There were also moderate increases in serum ferritin and transferrin saturation.


Similar content being viewed by others
References
Lozano R, Naghavi M, Foreman K et al (2012) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2095–2128
US Renal Data System (2013) USRDS 2013 Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda
Thodis ED, Oreopoulos DG (2011) Home dialysis first: a new paradigm for new ESRD patients. J Nephrol 24:398–404
Coladonato JA (2005) Control of hyperphosphatemia among patients with ESRD. J Am Soc Nephrol 16(Suppl 2):S107–S114
Qunibi WY (2004) Consequences of hyperphosphatemia in patients with end-stage renal disease (ESRD). Kidney Int Suppl 90:S8–S12
Kusano E (2011) Mechanism by which chronic kidney disease causes cardiovascular disease and the measures to manage this phenomenon. Clin Exp Nephrol 15:627–633
Miller LM, Sood MM, Sood AR et al (2010) Cardiovascular disease in end-stage renal disease: the challenge of assessing and managing cardiac disease in dialysis patients. Int Urol Nephrol 42:1007–1014
Qunibi W, Kalantar-Zadeh K (2011) Target levels for serum phosphorus and parathyroid hormone. Semin Dial 24:29–33
Gutiérrez OM, Wolf M (2010) Dietary phosphorus restriction in advanced chronic kidney disease: merits, challenges, and emerging strategies. Semin Dial 23:401–406
Tonelli M, Pannu N, Manns B (2010) Oral phosphate binders in patients with kidney failure. N Engl J Med 362:1312–1324
Hutchison AJ, Smith CP, Brenchley PE (2011) Pharmacology, efficacy and safety of oral phosphate binders. Nat Rev Nephrol 7:578–589
Yang WC, Yang CS, Hou CC, Wu TH, Young EW, Hsu CH (2002) An open-label, crossover study of a new phosphate-binding agent in haemodialysis patients: ferric citrate. Nephrol Dial Transplant 17:265–270
Dwyer JP, Sika M, Schulman G et al (2013) Dose-response and efficacy of ferric citrate to treat hyperphosphatemia in hemodialysis patients: a short-term randomized trial. Am J Kidney Dis 61:759–766
Sinsakul M, Sika M, Koury M et al (2012) The safety and tolerability of ferric citrate as a phosphate binder in dialysis patients. Nephron Clin Pract 121:c25–c29
Yokoyama K, Hirakata H, Akiba T, Sawada K, Kumagai Y (2012) Effect of oral JTT-751 (ferric citrate) on hyperphosphatemia in hemodialysis patients: results of a randomized, double-blind, placebo-controlled trial. Am J Nephrol 36:478–487
Yokoyama K, Akiba T, Fukagawa M et al (2014) A randomized trial of JTT-751 versus sevelamer hydrochloride in patients on hemodialysis. Nephrol Dial Transplant 29(5):1053–1060
Panion & BF Biotech, Inc. (2006) A randomized, double-blind, placebo-controlled, dose-ranging study of the effects of ferric citrate on serum phosphate in patients with end stage renal disease (ESRD). Panion & BF Biotech, Inc., Report No. PBB00101
Babitt JL, Lin HY (2012) Mechanisms of anemia in CKD. J Am Soc Nephrol 23:1631–1634
Calvo MS, Uribarri J (2013) Contributions to total phosphorus intake: all sources considered. Semin Dial 26:54–61
Hsu CH, Patel SR, Young EW (1999) New phosphate binding agents: ferric compounds. J Am Soc Nephrol 10:1274–1280
Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group (2012) KDIGO Clinical Practice Guideline for anemia in chronic kidney disease. Kidney Int 2(Suppl):279–335
Fishbane S (2008) Upper limit of serum ferritin: misinterpretation of the 2006 KDOQI anemia guidelines. Semin Dial 21:217–220
Acknowledgement
We would like to thank all participating investigators including Kuo CC, Wu CH, Ng HY, and Chiou YT (Chang Gung Memorial Hospital, Kaohsiung, Taiwan); Lee CC, Sun CY, Hsu HJ, and Tsai CJ (Chang Gung Medical Hospital, Keelung, Taiwan); Leu JG, and Lu LC (Shin Kong Wu Ho-Su Memorial Hospital, Taiwan); Hsu SP, Pai MF, Wu HY, and Chen HY (Far Eastern Memorial Hospital, Taiwan); Lin BS as the Medical Monitor, and Panion & BF Biotech Inc. as the sponsor of the study.
Conflict of interest
All authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
ClinicalTrials.gov; identifier: NCT01503736.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, CT., Wu, IW., Chiang, SS. et al. Effect of oral ferric citrate on serum phosphorus in hemodialysis patients: multicenter, randomized, double-blind, placebo-controlled study. J Nephrol 28, 105–113 (2015). https://doi.org/10.1007/s40620-014-0108-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-014-0108-6