Abstract
Background and Objectives
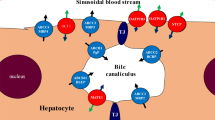
Understanding transmembrane transport provides a more complete understanding of the pharmacokinetics of a drug and mechanistic explanations for drug–drug interactions. Here, the transmembrane transport of danoprevir (hepatitis C virus protease inhibitor) and the effects of ritonavir and ciclosporin on transmembrane transport of danoprevir were evaluated and clinical pharmacokinetic studies of danoprevir co-administered with/without ritonavir and ciclosporin were conducted.
Methods
Transcellular transport of danoprevir was evaluated in Lewis lung cancer porcine kidney, Madin-Darby canine kidney, or Chinese hamster ovary cells transfected with human transport proteins, and in human hepatocytes. The pharmacokinetics of intravenous and oral danoprevir administered with/without ritonavir, and the impact of ciclosporin on danoprevir pharmacokinetics were evaluated in randomized, open-label, crossover studies in healthy subjects.
Results
Danoprevir transport in vitro involved organic anion transporting polypeptide (OATP) 1B1, OATP1B3, P-glycoprotein, and multidrug resistance protein-2, but not breast cancer resistance protein. Ritonavir and ciclosporin inhibited transport of danoprevir by human hepatocytes. The pharmacokinetics of intravenous danoprevir 6 mg were not altered by oral ritonavir 100 mg. In contrast, exposure to oral danoprevir 100 mg increased two- to threefold when co-administered with ritonavir. Absolute bioavailability of danoprevir 100 mg was low (1.15 %), but increased more than threefold (3.86 %) when co-administered with ritonavir. Oral ciclosporin 100 mg increased exposure to intravenous danoprevir 2 mg and oral ritonavir 100 mg.
Conclusion
Collectively, these studies provide insight into the transmembrane transport and pharmacokinetics of danoprevir and the mechanisms that underlie a recently reported, three-way drug–drug interaction involving danoprevir, ritonavir, and ciclosporin.




Similar content being viewed by others
References
Marcellin P, Cooper C, Balart L, Larrey D, Box T, Yoshida E, et al. Randomized controlled trial of danoprevir plus peginterferon alfa-2a and ribavirin in treatment-naive patients with hepatitis C virus genotype 1 infection. Gastroenterology. 2013;145:790–800.
Everson G, Cooper C, Hezode C, Shiffman ML, Yoshida E, Beltran-Jaramillo T, et al. DAUPHINE: a randomized phase II study of danoprevir/ritonavir plus peginterferon alpha-2a/ribavirin in HCV genotypes 1 or 4. Liver Int. 2014. doi:10.1111/liv.12471.
Gane EJ, Roberts SK, Stedman CA, Angus PW, Ritchie B, Elston R, et al. Oral combination therapy with a nucleoside polymerase inhibitor (RG7128) and danoprevir for chronic hepatitis C genotype 1 infection (INFORM-1): a randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet. 2010;376:1467–75.
Jensen DM, Brunda M, Elston R, Gane EJ, George J, Glavini K, et al. Interferon-free regimen containing setrobuvir (STV) in combination with ritonavir-boosted danoprevir (DNVr) and ribavirin (R) with or without mericitabine (MCB) in HCV genotype (G)1 treatment-naive patients: SVR4 results from the ANNAPURNA study (abstract 1098). Hepatology. 2013;58(Suppl):741A.
Feld JJ, Jacobson IM, Jensen DM, Foster GR, Pol S, Tam E, et al. Randomised study of danoprevir/ritonavir-based therapy for HCV genotype 1 patients with prior partial or null responses to peginterferon/ribavirin. J Hepatol. 2014. doi:10.1016/j.jhep.2014.09.013.
Gane EJ, Rouzier R, Wiercinska-Drapalo A, Larrey DG, Morcos PN, Brennan BJ, et al. Efficacy and safety of danoprevir-ritonavir plus peginterferon alfa-2a-ribavirin in hepatitis C virus genotype 1 prior null responders. Antimicrob Agents Chemother. 2014;58:1136–45.
Gane EJ, Rouzier R, Stedman C, Wiercinska-Drapalo A, Horban A, Chang L, et al. Antiviral activity, safety, and pharmacokinetics of danoprevir/ritonavir plus PEG-IFN alpha-2a/RBV in hepatitis C patients. J Hepatol. 2011;55:972–9.
Forestier N, Larrey D, Guyader D, Marcellin P, Rouzier R, Patat A, et al. Treatment of chronic hepatitis C patients with the NS3/4A protease inhibitor danoprevir (ITMN-191/RG7227) leads to robust reductions in viral RNA: a phase 1b multiple ascending dose study. J Hepatol. 2011;54:1130–6.
Morcos PN, Kulkarni R, Scoon S, Smith PF, Brennan BJ. Effect of ritonavir-boosted danoprevir, a potent hepatitis C virus protease inhibitor, on QTc interval in healthy subjects: results from a thorough QT study. Drug Dev Res. 2013;74:306–15.
Brennan BJ, Moreira SA, Morcos PN, Navarro MT, Asthappan J, Goelzer P, et al. Pharmacokinetics of a three-way drug interaction between danoprevir, ritonavir and the organic anion transporting polypeptide (OATP) inhibitor ciclosporin. Clin Pharmacokinet. 2013;52:805–13.
Wu CY, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22:11–23.
Shugarts S, Benet LZ. The role of transporters in the pharmacokinetics of orally administered drugs. Pharm Res. 2009;26:2039–54.
Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–36.
Hillgren KM, Keppler D, Zur AA, Giacomini KM, Stieger B, Cass CE, et al. Emerging transporters of clinical importance: an update from the International Transporter Consortium. Clin Pharmacol Ther. 2013;94:52–63.
Tweedie D, Polli JW, Berglund EG, Huang SM, Zhang L, Poirier A, et al. Transporter studies in drug development: experience to date and follow-up on decision trees from the International Transporter Consortium. Clin Pharmacol Ther. 2013;94:113–25.
Poirier A, Funk C, Lave T, Noe J. New strategies to address drug-drug interactions involving OATPs. Curr Opin Drug Discov Devel. 2007;10:74–83.
Poirier A, Lave T, Portmann R, Brun ME, Senner F, Kansy M, et al. Design, data analysis, and simulation of in vitro drug transport kinetic experiments using a mechanistic in vitro model. Drug Metab Dispos. 2008;36:2434–44.
Schwab D, Fischer H, Tabatabaei A, Poli S, Huwyler J. Comparison of in vitro P-glycoprotein screening assays: recommendations for their use in drug discovery. J Med Chem. 2003;46:1716–25.
Paine SW, Parker AJ, Gardiner P, Webborn PJ, Riley RJ. Prediction of the pharmacokinetics of atorvastatin, cerivastatin, and indomethacin using kinetic models applied to isolated rat hepatocytes. Drug Metab Dispos. 2008;36:1365–74.
Poirier A, Cascais AC, Funk C, Lave T. Prediction of pharmacokinetic profile of valsartan in human based on in vitro uptake transport data. J Pharmacokinet Pharmacodyn. 2009;36:585–611.
Webborn PJ, Parker AJ, Denton RL, Riley RJ. In vitro-in vivo extrapolation of hepatic clearance involving active uptake: theoretical and experimental aspects. Xenobiotica. 2007;37:1090–109.
Zamek-Gliszczynski MJ, Lee CA, Poirier A, Bentz J, Chu X, Ellens H, et al. ITC recommendations for transporter kinetic parameter estimation and translational modeling of transport-mediated PK and DDIs in humans. Clin Pharmacol Ther. 2013;94:64–79.
Annaert P, Ye ZW, Stieger B, Augustijns P. Interaction of HIV protease inhibitors with OATP1B1, 1B3, and 2B1. Xenobiotica. 2010;40:163–76.
Melchior DL, Sharom FJ, Evers R, Wright GE, Chu JW, Wright SE, et al. Determining P-glycoprotein-drug interactions: evaluation of reconstituted P-glycoprotein in a liposomal system and LLC-MDR1 polarized cell monolayers. J Pharmacol Toxicol Methods. 2012;65:64–74.
Letschert K, Faulstich H, Keller D, Keppler D. Molecular characterization and inhibition of amanitin uptake into human hepatocytes. Toxicol Sci. 2006;91:140–9.
Sugimoto H, Matsumoto S, Tachibana M, Niwa S, Hirabayashi H, Amano N, et al. Establishment of in vitro P-glycoprotein inhibition assay and its exclusion criteria to assess the risk of drug-drug interaction at the drug discovery stage. J Pharm Sci. 2011;100:4013–23.
Tang F, Horie K, Borchardt RT. Are MDCK cells transfected with the human MRP2 gene a good model of the human intestinal mucosa? Pharm Res. 2002;19:773–9.
Amundsen R, Asberg A, Ohm IK, Christensen H. Cyclosporine A- and tacrolimus-mediated inhibition of CYP3A4 and CYP3A5 in vitro. Drug Metab Dispos. 2012;40:655–61.
McGinnity DF, Zhang G, Kenny JR, Hamilton GA, Otmani S, Stams KR, et al. Evaluation of multiple in vitro systems for assessment of CYP3A4 induction in drug discovery: human hepatocytes, pregnane X receptor reporter gene, and Fa2N-4 and HepaRG cells. Drug Metab Dispos. 2009;37:1259–68.
Kaminsky LS, Zhang QY. The small intestine as a xenobiotic-metabolizing organ. Drug Metab Dispos. 2003;31:1520–5.
Zhou SF. Structure, function and regulation of P-glycoprotein and its clinical relevance in drug disposition. Xenobiotica. 2008;38:802–32.
Morcos PN, Chang L, Navarro M, Chung D, Smith PF, Brennan BJ, et al. Two-way interaction study between ritonavir-boosted danoprevir, a potent HCV protease inhibitor, and ketoconazole in healthy subjects. Int J Clin Pharmacol Ther. 2014;52:103–11.
Dobson EL, Warner GF, Finney CR, Johnston ME. The measurement of liver circulation by means of the colloid disappearance rate. I. Liver blood flow in normal young men. Circulation. 1953;7:690–5.
Lau YY, Okochi H, Huang Y, Benet LZ. Pharmacokinetics of atorvastatin and its hydroxy metabolites in rats and the effects of concomitant rifampicin single doses: relevance of first-pass effect from hepatic uptake transporters, and intestinal and hepatic metabolism. Drug Metab Dispos. 2006;34:1175–81.
Morcos PN, Chang L, Kulkarni R, Giraudon M, Shulman N, Brennan BJ, et al. A randomised study of the effect of danoprevir/ritonavir or ritonavir on substrates of cytochrome P450 (CYP) 3A and 2C9 in chronic hepatitis C patients using a drug cocktail. Eur J Clin Pharmacol. 2013;69:1939–49.
Morcos PN, Moreira SA, Navarro MT, Bech N, Quatkemeyer A, Smith PF, et al. Effect of meal and antisecretory agents on the pharmacokinetics of danoprevir/ritonavir in healthy volunteers. J Pharm Pharmacol. 2014;66:23–31.
Acknowledgments
BB, SM, PM, PG, JA, and PS are/were employed by Hoffmann La-Roche Inc., Nutley, NJ, USA, at the time the study was conducted. AP, RP, and CF are/were employed by F. Hoffmann-La Roche Ltd., Basel, Switzerland, at the time the study was conducted. This study was supported by F. Hoffmann-La Roche Ltd. Support for third-party writing assistance for this manuscript, furnished by Blair Jarvis, was provided by F. Hoffmann-La Roche Ltd.
Conflict of interest
All authors were employees of F. Hoffmann-La Roche Ltd. or Hoffmann-La Roche Inc. at the time this research was conducted.
Ethics
The protocol and amendments were reviewed and approved by an independent ethics committee. The study was conducted in accordance with the principles of the Declaration of Helsinki and in compliance with the International Conference on Harmonisation Guideline for Good Clinical Practice (CPMP/ICH/135/95) and the European Union Common Technical Document: Directive 2001/20/EC. All participants provided informed written consent prior to undergoing any study procedures.
Author contributions
All authors critically reviewed and revised the manuscript for intellectual content.
BB—designed research, performed research, acquisition of data, analysis and interpretation of data.
AP—designed research, acquisition of data, analysis and interpretation of data.
SM—performed research, analysis and interpretation of data, revision of manuscript.
PM—designed the research, analysis and interpretation of data, revision of manuscript.
PG—designed research and integrated data.
RP—performed research, acquisition of data, analysis and interpretation of data.
JA—designed research, acquisition of data.
CF—analysis and interpretation of data.
PS—designed research, analysis and interpretation of data.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brennan, B.J., Poirier, A., Moreira, S. et al. Characterization of the Transmembrane Transport and Absolute Bioavailability of the HCV Protease Inhibitor Danoprevir. Clin Pharmacokinet 54, 537–549 (2015). https://doi.org/10.1007/s40262-014-0222-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-014-0222-6