Abstract
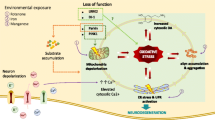
Parkinson’s disease (PD) is a progressive neurodegenerative disorder that is characterized by (1) the selective loss of dopaminergic neurons in the substantia nigra and (2) the deposition of misfolded α-synuclein (α-syn) as amyloid fibrils in the intracellular Lewy bodies in various region of the brain. Current thinking suggests that an interaction between α-syn and dopamine (DA) leads to the selective death of neuronal cells and the accumulation of misfolded α-syn. However, the exact mechanism by which this occurs is not fully defined. DA oxidation could play a key role is the pathogenesis of PD by causing oxidative stress, mitochondria dysfunction and impairment of protein metabolism. Here, we review the literature on the role of DA and its oxidative intermediates in modulating the aggregation pathways of α-syn.


Similar content being viewed by others
References
Lotharius J, Brundin P (2002) Pathogenesis of Parkinson’s disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci 3:932–942. doi:10.1038/nrn983
Thomas B, Beal MF (2007) Parkinson’s disease. Hum Mol Genet 16(Review issue 2):R183–R194. doi:10.1093/hmg/ddm159
Pakkenberg B, Moller A, Gundersen HJ et al (1991) The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson’s disease estimated with an unbiased stereological method. J Neurol Neurosurg Psychiatry 54:30–33. doi:10.1136/jnnp.54.1.30
Braak H, Del Tredici K, Rub U et al (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211. doi:10.1016/S0197-4580(02)00065-9
Spillantini MG, Schmidt ML, Lee VM et al (1997) Alpha-synuclein in Lewy bodies. Nature 388:839–840. doi:10.1038/42166
Spillantini MG, Crowther RA, Jakes R et al (1998) Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci USA 95:6469–6473. doi:10.1073/pnas.95.11.6469
Wakabayashi K, Tanji K, Mori F, Takahashi H (2007) The Lewy body in Parkinson’s disease: molecules implicated in the formation and degradation of alpha-synuclein aggregates. Neuropathology 27:494–506. doi:10.1111/j.1440-1789.2007.00803.x
Polymeropoulos MH, Lavedan C, Leroy E et al (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276:2045–2047. doi:10.1126/science.276.5321.2045
Kruger R, Kuhn W, Muller T et al (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet 18:106–108. doi:10.1038/ng0298-106
Singleton AB, Farrer M, Johnson J et al (2003) Alpha-synuclein locus triplication causes Parkinson’s disease. Science 302:841. doi:10.1126/science.1090278
Zarranz JJ, Alegre J, Gomez-Esteban JC et al (2004) The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55:164–173. doi:10.1002/ana.10795
Chartier-Harlin MC, Kachergus J, Roumier C et al (2004) Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364:1167–1169. doi:10.1016/S0140-6736(04)17103-1
Davidson WS, Jonas A, Clayton DF et al (1998) Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem 273:9443–9449. doi:10.1074/jbc.273.16.9443
Eliezer D, Kutluay E, Bussell R Jr et al (2001) Conformational properties of alpha-synuclein in its free and lipid-associated states. J Mol Biol 307:1061–1073. doi:10.1006/jmbi.2001.4538
Chandra S, Chen X, Rizo J et al (2003) A broken alpha-helix in folded alpha-synuclein. J Biol Chem 278:15313–15318. doi:10.1074/jbc.M213128200
Ueda K, Fukushima H, Masliah E et al (1993) Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci USA 90:11282–11286. doi:10.1073/pnas.90.23.11282
Culvenor JG, McLean CA, Cutt S et al (1999) Non-Abeta component of Alzheimer’s disease amyloid (NAC) revisited. NAC and alpha-synuclein are not associated with Abeta amyloid. Am J Pathol 155:1173–1181
Giasson BI, Murray IV, Trojanowski JQ et al (2001) A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem 276:2380–2386. doi:10.1074/jbc.M008919200
Perez RG, Waymire JC, Lin E et al (2002) A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci 22:3090–3099
Giros B, Caron MG (1993) Molecular characterization of the dopamine transporter. Trends Pharmacol Sci 14:43–49. doi:10.1016/0165-6147(93)90029-J
Abeliovich A, Schmitz Y, Farinas I et al (2000) Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25:239–252. doi:10.1016/S0896-6273(00)80886-7
Larsen KE, Schmitz Y, Troyer MD et al (2006) Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci 26:11915–11922. doi:10.1523/JNEUROSCI.3821-06.2006
Galvin JE (2006) Interaction of alpha-synuclein and dopamine metabolites in the pathogenesis of Parkinson’s disease: a case for the selective vulnerability of the substantia nigra. Acta Neuropathol 112:115–126. doi:10.1007/s00401-006-0096-2
Sulzer D, Bogulavsky J, Larsen KE et al (2000) Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc Natl Acad Sci USA 97:11869–11874. doi:10.1073/pnas.97.22.11869
Graham DG, Tiffany SM, Bell WR et al (1978) Autoxidation versus covalent binding of quinones as the mechanism of toxicity of dopamine, 6-hydroxydopamine, and related compounds toward C1300 neuroblastoma cells in vitro. Mol Pharmacol 14:644–653
Zecca L, Zucca FA, Wilms H et al (2003) Neuromelanin of the substantia nigra: a neuronal black hole with protective and toxic characteristics. Trends Neurosci 26:578–580. doi:10.1016/j.tins.2003.08.009
Hirsch E, Graybiel AM, Agid YA (1988) Melanized dopaminergic neurons are differentially susceptible to degeneration in parkinson’s disease. Nature 334:345–348. doi:10.1038/334345a0
Bisaglia M, Mammi S, Bubacco L (2007) Kinetic and structural analysis of the early oxidation products of dopamine: analysis of the interactions with alpha-synuclein. J Biol Chem 282:15597–15605. doi:10.1074/jbc.M610893200
Li SW, Lin TS, Minteer S et al (2001) 3, 4-dihydroxyphenylacetaldehyde and hydrogen peroxide generate a hydroxyl radical: possible role in Parkinson’s disease pathogenesis. Brain Res Mol Brain Res 93:1–7. doi:10.1016/S0169-328X(01)00120-6
Pham CLL, Leong SL, Ali FE et al (2009) Dopamine and the dopamine oxidation product 5, 6-dihydroxylindole promote distinct on-pathway and off-pathway aggregation of α-synuclein in a ph-dependent manner. J Mol Biol 387:771–785. doi:10.1016/j.jmb.2009.02.007
Conway KA, Harper JD, Lansbury PT (1998) Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med 4:1318–1320. doi:10.1038/3311
Wood SJ, Wypych J, Steavenson S et al (1999) Alpha-synuclein fibrillogenesis is nucleation-dependent. Implications for the pathogenesis of Parkinson’s disease. J Biol Chem 274:19509–19512. doi:10.1074/jbc.274.28.19509
Harper JD, Lansbury PT Jr (1997) Models of amyloid seeding in alzheimer’s disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu Rev Biochem 66:385–407. doi:10.1146/annurev.biochem.66.1.385
Uversky VN (2007) Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. J Neurochem 103:17–37
Uversky VN, Li J, Fink AL (2001) Evidence for a partially folded intermediate in alpha-synuclein fibril formation. J Biol Chem 276:10737–10744. doi:10.1074/jbc.M010907200
Uversky VN, Lee HJ, Li J et al (2001) Stabilization of partially folded conformation during alpha-synuclein oligomerization in both purified and cytosolic preparations. J Biol Chem 276:43495–43498. doi:10.1074/jbc.C100551200
Hoyer W, Antony T, Cherny D et al (2002) Dependence of alpha-synuclein aggregate morphology on solution conditions. J Mol Biol 322:383–393. doi:10.1016/S0022-2836(02)00775-1
Uversky VN, Li J, Fink AL (2001) Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson’s disease and heavy metal exposure. J Biol Chem 276:44284–44296. doi:10.1074/jbc.M105343200
Uversky VN, Yamin G, Souillac PO et al (2002) Methionine oxidation inhibits fibrillation of human alpha-synuclein in vitro. FEBS Lett 517:239–244. doi:10.1016/S0014-5793(02)02638-8
Leong SL, Pham CLL, Galatis D et al (2009) Formation of dopamine-mediated alpha-synuclein-soluble oligomers requires methionine oxidation. Free Radic Biol Med 46:1328–1337. doi:10.1016/j.freeradbiomed.2009.02.009
Uversky VN, Yamin G, Munishkina LA et al (2005) Effects of nitration on the structure and aggregation of alpha-synuclein. Brain Res Mol Brain Res 134:84–102. doi:10.1016/j.molbrainres.2004.11.014
Yamin G, Uversky VN, Fink AL (2003) Nitration inhibits fibrillation of human alpha-synuclein in vitro by formation of soluble oligomers. FEBS Lett 542:147–152. doi:10.1016/S0014-5793(03)00367-3
Kayed R, Head E, Thompson JL et al (2003) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300:486–489. doi:10.1126/science.1079469
Bucciantini M, Giannoni E, Chiti F et al (2002) Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 416:507–511. doi:10.1038/416507a
Caughey B, Lansbury PT (2003) Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci 26:267–298. doi:10.1146/annurev.neuro.26.010302.081142
Lambert MP, Barlow AK, Chromy BA et al (1998) Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA 95:6448–6453. doi:10.1073/pnas.95.11.6448
Conway KA, Lee SJ, Rochet JC et al (2000) Accelerated oligomerization by Parkinson’s disease linked alpha-synuclein mutants. Ann N Y Acad Sci 920:42–45
Conway KA, Harper JD, Lansbury PT Jr (2000) Fibrils formed in vitro from alpha-synuclein and two mutant forms linked to Parkinson’s disease are typical amyloid. Biochemistry 39:2552–2563. doi:10.1021/bi991447r
Greenbaum EA, Graves CL, Mishizen-Eberz AJ et al (2005) The E46K mutation in alpha-synuclein increases amyloid fibril formation. J Biol Chem 280:7800–7807. doi:10.1074/jbc.M411638200
Smith DP, Tew DJ, Hill AF et al (2008) Formation of a high affinity lipid-binding intermediate during the early aggregation phase of alpha-synuclein. Biochemistry 47:1425–1434. doi:10.1021/bi701522m
Tabrizi SJ, Orth M, Wilkinson JM et al (2000) Expression of mutant alpha-synuclein causes increased susceptibility to dopamine toxicity. Hum Mol Genet 9:2683–2689. doi:10.1093/hmg/9.18.2683
Narhi L, Wood SJ, Steavenson S et al (1999) Both familial Parkinson’s disease mutations accelerate alpha-synuclein aggregation. J Biol Chem 274:9843–9846. doi:10.1074/jbc.274.14.9843
Conway KA, Lee SJ, Rochet JC et al (2000) Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proc Natl Acad Sci USA 97:571–576. doi:10.1073/pnas.97.2.571
Li J, Uversky VN, Fink AL (2001) Effect of familial Parkinson’s disease point mutations A30P and A53T on the structural properties, aggregation, and fibrillation of human alpha-synuclein. Biochemistry 40:11604–11613. doi:10.1021/bi010616g
Rochet JC, Conway KA, Lansbury PT Jr (2000) Inhibition of fibrillization and accumulation of prefibrillar oligomers in mixtures of human and mouse alpha-synuclein. Biochemistry 39:10619–10626. doi:10.1021/bi001315u
Lashuel HA, Hartley D, Petre BM et al (2002) Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature 418:291. doi:10.1038/418291a
Lashuel HA, Petre BM, Wall J et al (2002) Alpha-synuclein, especially the parkinson’s disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol 322:1089–1102. doi:10.1016/S0022-2836(02)00735-0
Volles MJ, Lansbury PT Jr (2002) Vesicle permeabilization by protofibrillar alpha-synuclein is sensitive to Parkinson’s disease-linked mutations and occurs by a pore-like mechanism. Biochemistry 41:4595–4602. doi:10.1021/bi0121353
Pountney DL, Voelcker NH, Gai WP (2005) Annular alpha-synuclein oligomers are potentially toxic agents in alpha-synucleinopathy. Hypothesis. Neurotox Res 7:59–67
Danzer KM, Haasen D, Karow AR et al (2007) Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci 27:9220–9232. doi:10.1523/JNEUROSCI.2617-07.2007
Emmanouilidou E, Stefanis L, Vekrellis K (2008) Cell-produced alpha-synuclein oligomers are targeted to, and impair, the 26S proteasome. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2008.07.008
Cappai R, Leck SL, Tew DJ et al (2005) Dopamine promotes alpha-synuclein aggregation into SDS-resistant soluble oligomers via a distinct folding pathway. FASEB J 19:1377–1379
Li J, Zhu M, Manning-Bog AB et al (2004) Dopamine and L-dopa disaggregate amyloid fibrils: implications for Parkinson’s and Alzheimer’s disease. FASEB J 18:962–964. doi:10.1096/fj.04-2273com
Norris EH, Giasson BI, Hodara R et al (2005) Reversible inhibition of alpha-synuclein fibrillization by dopaminochrome-mediated conformational alterations. J Biol Chem 280:21212–21219. doi:10.1074/jbc.M412621200
Kuhn DM, Arthur RE Jr, Thomas DM et al (1999) Tyrosine hydroxylase is inactivated by catechol-quinones and converted to a redox-cycling quinoprotein: possible relevance to Parkinson’s disease. J Neurochem 73:1309–1317. doi:10.1046/j.1471-4159.1999.0731309.x
Xu Y, Stokes AH, Roskoski R Jr et al (1998) Dopamine, in the presence of tyrosinase, covalently modifies and inactivates tyrosine hydroxylase. J Neurosci Res 54:691–697. doi:10.1002/(SICI)1097-4547(19981201)54:5<691::AID-JNR14>3.0.CO;2-F
Whitehead RE, Ferrer JV, Javitch JA et al (2001) Reaction of oxidized dopamine with endogenous cysteine residues in the human dopamine transporter. J Neurochem 76:1242–1251. doi:10.1046/j.1471-4159.2001.00125.x
Moussa CE, Mahmoodian F, Tomita Y et al (2008) Dopamine differentially induces aggregation of A53T mutant and wild type alpha-synuclein: insights into the protein chemistry of Parkinson’s disease. Biochem Biophys Res Commun 365:833–839. doi:10.1016/j.bbrc.2007.11.075
Mazzulli JR, Mishizen AJ, Giasson BI et al (2006) Cytosolic catechols inhibit alpha-synuclein aggregation and facilitate the formation of intracellular soluble oligomeric intermediates. J Neurosci 26:10068–10078. doi:10.1523/JNEUROSCI.0896-06.2006
Follmer C, Romao L, Einsiedler CM et al (2007) Dopamine affects the stability, hydration, and packing of protofibrils and fibrils of the wild type and variants of alpha-synuclein. Biochemistry 46:472–482. doi:10.1021/bi061871+
Conway KA, Rochet JC, Bieganski RM et al (2001) Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science 294:1346–1349. doi:10.1126/science.1063522
Li HT, Lin DH, Luo XY et al (2005) Inhibition of alpha-synuclein fibrillization by dopamine analogs via reaction with the amino groups of alpha-synuclein. Implication for dopaminergic neurodegeneration. FEBS J 272:3661–3672. doi:10.1111/j.1742-4658.2005.04792.x
LaVoie MJ, Ostaszewski BL, Weihofen A et al (2005) Dopamine covalently modifies and functionally inactivates Parkin. Nat Med 11:1214–1221. doi:10.1038/nm1314
Gotz ME, Double K, Gerlach M et al (2004) The relevance of iron in the pathogenesis of Parkinson’s disease. Ann N Y Acad Sci 1012:193–208. doi:10.1196/annals.1306.017
Burke WJ, Kumar VB, Pandey N et al (2008) Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol 115:193–203. doi:10.1007/s00401-007-0303-9
Zhou W, Gallagher A, Hong DP et al (2009) At low concentrations, 3,4-dihydroxyphenylacetic acid (DOPAC) binds non-covalently to alpha-synuclein and prevents its fibrillation. J Mol Biol 388:597–610. doi:10.1016/j.jmb.2009.03.053
Mazzulli JR, Armakola M, Dumoulin M et al (2007) Cellular oligomerization of alpha-synuclein is determined by the interaction of oxidized catechols with a C-terminal sequence. J Biol Chem 282:31621–31630. doi:10.1074/jbc.M704737200
Herrera FE, Chesi A, Paleologou KE et al (2008) Inhibition of alpha-synuclein fibrillization by dopamine is mediated by interactions with five C-terminal residues and with E83 in the NAC region. PLoS ONE 3:E3394. doi:10.1371/journal.pone.0003394
Hokenson MJ, Uversky VN, Goers J et al (2004) Role of individual methionines in the fibrillation of methionine-oxidized alpha-synuclein. Biochemistry 43:4621–4633. doi:10.1021/bi049979h
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leong, S.L., Cappai, R., Barnham, K.J. et al. Modulation of α-Synuclein Aggregation by Dopamine: A Review. Neurochem Res 34, 1838–1846 (2009). https://doi.org/10.1007/s11064-009-9986-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-009-9986-8