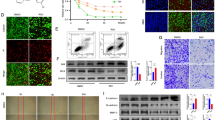
Abstract
The present study was undertaken to determine the molecular mechanism by which kaempferol induces cell death in human glioma cells. Kaempferol resulted in loss of cell viability and inhibition of proliferation in a dose- and time-dependent manner, which were largely attributed to cell death. Kaempferol caused an increase in reactive oxygen species (ROS) generation and the kaempferol-induced cell death was prevented by antioxidants, suggesting that ROS generation is involved in kaempferol-induced cell death. Kaempferol caused depolarization of mitochondrial membrane potential. Western blot analysis showed that kaempferol treatment caused a rapid reduction in phosphorylation of extracellular signal-regulated kinase (ERK) and Akt. The ERK inhibitor U0126 and the Akt inhibitor LY984002 increased the kaempferol-induced cell death and overexpression of MEK, the upstream kinase of ERK, and Akt prevented the cell death. The expression of anti-apoptotic proteins XIAP and survivin was down-regulated by kaempferol and its effect was prevented by overexpression of MEK and Akt. Kaempferol induced activation of caspase-3 and kaempferol-induced cell death was prevented by caspase inhibitors. Taken together, these findings suggest that kaempferol results in human glioma cell death through caspase-dependent mechanisms involving down-regulation of XIAP and survivin regulating by ERK and Akt.






Similar content being viewed by others
References
Ohgaki H, Kleihues P (2005) Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol 64(6):479–489
DeAngelis LM (2001) Brain tumors. N Engl J Med 344(2):114–123. doi:10.1056/NEJM200101113440207
Middleton E Jr, Kandaswami C, Theoharides TC (2000) The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 52(4):673–751
Yang JH, Hsia TC, Kuo HM, Chao PD, Chou CC, Wei YH, Chung JG (2006) Inhibition of lung cancer cell growth by quercetin glucuronides via G2/M arrest and induction of apoptosis. Drug Metab Dispos 34(2):296–304. doi:10.1124/dmd.105.005280
Kanadaswami C, Lee LT, Lee PP, Hwang JJ, Ke FC, Huang YT, Lee MT (2005) The antitumor activities of flavonoids. In Vivo 19(5):895–909
Ren W, Qiao Z, Wang H, Zhu L, Zhang L (2003) Flavonoids: promising anticancer agents. Med Res Rev 23(4):519–534. doi:10.1002/med.10033
Fresco P, Borges F, Diniz C, Marques MP (2006) New insights on the anticancer properties of dietary polyphenols. Med Res Rev 26(6):747–766. doi:10.1002/med.20060
Braganhol E, Zamin LL, Canedo AD, Horn F, Tamajusuku AS, Wink MR, Salbego C, Battastini AM (2006) Antiproliferative effect of quercetin in the human U138MG glioma cell line. Anticancer Drugs 17(6):663–671. doi:10.1097/01.cad.0000215063.23932.02
Shen SC, Lin CW, Lee HM, Chien LL, Chen YC (2006) Lipopolysaccharide plus 12-o-tetradecanoylphorbol 13-acetate induction of migration and invasion of glioma cells in vitro and in vivo: Differential inhibitory effects of flavonoids. Neuroscience 140(2):477–489. doi:10.1016/j.neuroscience.2006.02.028
Sharma V, Joseph C, Ghosh S, Agarwal A, Mishra MK, Sen E (2007) Kaempferol induces apoptosis in glioblastoma cells through oxidative stress. Mol Cancer Ther 6(9):2544–2553. doi:10.1158/1535-7163.MCT-06-0788
Leung HW, Lin CJ, Hour MJ, Yang WH, Wang MY, Lee HZ (2007) Kaempferol induces apoptosis in human lung non-small carcinoma cells accompanied by an induction of antioxidant enzymes. Food Chem Toxicol 45(10):2005–2013. doi:10.1016/j.fct.2007.04.023
Nguyen TT, Tran E, Ong CK, Lee SK, Do PT, Huynh TT, Nguyen TH, Lee JJ, Tan Y, Ong CS, Huynh H (2003) Kaempferol-induced growth inhibition and apoptosis in A549 lung cancer cells is mediated by activation of MEK-MAPK. J Cell Physiol 197(1):110–121. doi:10.1002/jcp.10340
Dimas K, Demetzos C, Mitaku S, Marselos M, Tzavaras T, Kokkinopoulos D (2000) Cytotoxic activity of kaempferol glycosides against human leukaemic cell lines in vitro. Pharmacol Res 41(1):85–88. doi:10.1006/phrs.1999.0562
Denizot F, Lang R (1986) Rapid colorimetric assay for cell growth and survival Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 89(2):271–277. doi:10.1016/0022-1759(86)90368-6
Pastorino JG, Chen ST, Tafani M, Snyder JW, Farber JL (1998) The overexpression of Bax produces cell death upon induction of the mitochondrial permeability transition. J Biol Chem 273(13):7770–7775. doi:10.1074/jbc.273.13.7770
Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270(5240):1326–1331. doi:10.1126/science.270.5240.1326
Cobb MH (1999) MAP kinase pathways. Prog Biophys Mol Biol 71(3–4):479–500. doi:10.1016/S0079-6107(98)00056-X
Coffer PJ, Jin J, Woodgett JR (1998) Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J 335(Pt 1):1–13
Fukuda S, Pelus LM (2006) Survivin, a cancer target with an emerging role in normal adult tissues. Mol Cancer Ther 5(5):1087–1098. doi:10.1158/1535-7163.MCT-05-0375
Tamm I, Kornblau SM, Segall H, Krajewski S, Welsh K, Kitada S, Scudiero DA, Tudor G, Qui YH, Monks A, Andreeff M, Reed JC (2000) Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin Cancer Res 6(5):1796–1803
Cohen GM (1997) Caspases: the executioners of apoptosis. Biochem J 326:1–16
Miura YH, Tomita I, Watanabe T, Hirayama T, Fukui S (1998) Active oxygens generation by flavonoids. Biol Pharm Bull 21(2):93–96
Wang IK, Lin-Shiau SY, Lin JK (1999) Induction of apoptosis by apigenin and related flavonoids through cytochrome c release and activation of caspase-9 and caspase-3 in leukaemia HL-60 cells. Eur J Cancer 35(10):1517–1525. doi:10.1016/S0959-8049(99)00168-9
Chen YC, Shen SC, Chow JM, Ko CH, Tseng SW (2004) Flavone inhibition of tumor growth via apoptosis in vitro and in vivo. Int J Oncol 25(3):661–670
Pastorino JG, Snyder JW, Serroni A, Hoek JB, Farber JL (1993) Cyclosporin and carnitine prevent the anoxic death of cultured hepatocytes by inhibiting the mitochondrial permeability transition. J Biol Chem 268(19):13791–13798
Kroemer G, Dallaporta B, Resche-Rigon M (1998) The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol 60:619–642
Tatton WG, Olanow CW (1999) Apoptosis in neurodegenerative diseases: the role of mitochondria. Biochim Biophys Acta 1410(2):195–213. doi:10.1016/S0005-2728(98)00167-4
Bhaskara VK, Sundaram C, Babu PP (2006) pERK, pAkt and pBad: a possible role in cell proliferation and sustained cellular survival during tumorigenesis and tumor progression in ENU induced transplacental glioma rat model. Neurochem Res 31(9):1163–1170. doi:10.1007/s11064-006-9142-7
Jacques-Silva MC, Bernardi A, Rodnight R, Lenz G (2004) ERK, PKC and PI3 K/Akt pathways mediate extracellular ATP and adenosine-induced proliferation of U138-MG human glioma cell line. Oncology 67(5–6):450–459. doi:10.1159/000082930
Wang L, Liu F, Adamo ML (2001) Cyclic AMP inhibits extracellular signal-regulated kinase and phosphatidylinositol 3-kinase/Akt pathways by inhibiting Rap1. J Biol Chem 276(40):37242–37249. doi:10.1074/jbc.M105089200
Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22(2):153–183. doi:10.1210/er.22.2.153
Groom LA, Sneddon AA, Alessi DR, Dowd S, Keyse SM (1996) Differential regulation of the MAP, SAP and RK/p38 kinases by Pyst1, a novel cytosolic dual-specificity phosphatase. EMBO J 15(14):3621–3632
Kortenjann M, Thomae O, Shaw PE (1994) Inhibition of v-raf-dependent c-fos expression and transformation by a kinase-defective mutant of the mitogen-activated protein kinase Erk2. Mol Cell Biol 14(7):4815–4824
Brunet A, Pages G, Pouyssegur J (1994) Constitutively active mutants of MAP kinase kinase (MEK1) induce growth factor-relaxation and oncogenicity when expressed in fibroblasts. Oncogene 9(11):3379–3387
Moon SK, Cho GO, Jung SY, Gal SW, Kwon TK, Lee YC, Madamanchi NR, Kim CH (2003) Quercetin exerts multiple inhibitory effects on vascular smooth muscle cells: role of ERK1/2, cell-cycle regulation, and matrix metalloproteinase-9. Biochem Biophys Res Commun 301(4):1069–1078. doi:10.1016/S0006-291X(03)00091-3
Singh RP, Tyagi AK, Zhao J, Agarwal R (2002) Silymarin inhibits growth and causes regression of established skin tumors in SENCAR mice via modulation of mitogen-activated protein kinases and induction of apoptosis. Carcinogenesis 23(3):499–510. doi:10.1093/carcin/23.3.499
Spencer JP, Rice-Evans C, Williams RJ (2003) Modulation of pro-survival Akt/protein kinase B and ERK1/2 signaling cascades by quercetin and its in vivo metabolites underlie their action on neuronal viability. J Biol Chem 278(37):34783–34793. doi:10.1074/jbc.M305063200
Nguyen TT, Tran E, Nguyen TH, Do PT, Huynh TH, Huynh H (2004) The role of activated MEK-ERK pathway in quercetin-induced growth inhibition and apoptosis in A549 lung cancer cells. Carcinogenesis 25(5):647–659. doi:10.1093/carcin/bgh052
Agullo G, Gamet-Payrastre L, Manenti S, Viala C, Remesy C, Chap H, Payrastre B (1997) Relationship between flavonoid structure and inhibition of phosphatidylinositol 3-kinase: a comparison with tyrosine kinase and protein kinase C inhibition. Biochem Pharmacol 53(11):1649–1657. doi:10.1016/S0006-2952(97)82453-7
Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, Williams RL (2000) Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell 6(4):909–919. doi:10.1016/S1097-2765(05)00089-4
Granado-Serrano AB, Martin MA, Bravo L, Goya L, Ramos S (2006) Quercetin Induces Apoptosis via Caspase Activation, Regulation of Bcl-2, and Inhibition of PI-3-Kinase/Akt and ERK Pathways in a Human Hepatoma Cell Line (HepG2). J Nutr 136(11):2715–2721
Ni T, Li W, Zou F (2005) The ubiquitin ligase ability of IAPs regulates apoptosis. IUBMB Life 57(12):779–785. doi:10.1080/15216540500389013
Liston P, Young SS, Mackenzie AE, Korneluk RG (1997) Life and death decisions: the role of the IAPs in modulating programmed cell death. Apoptosis 2(5):423–441. doi:10.1023/A:1026465926478
Chakravarti A, Noll E, Black PM, Finkelstein DF, Finkelstein DM, Dyson NJ, Loeffler JS (2002) Quantitatively determined survivin expression levels are of prognostic value in human gliomas. J Clin Oncol 20(4):1063–1068. doi:10.1200/JCO.20.4.1063
Kajiwara Y, Yamasaki F, Hama S, Yahara K, Yoshioka H, Sugiyama K, Arita K, Kurisu K (2003) Expression of survivin in astrocytic tumors: correlation with malignant grade and prognosis. Cancer 97(4):1077–1083. doi:10.1002/cncr.11122
McLaughlin N, Annabi B, Bouzeghrane M, Temme A, Bahary JP, Moumdjian R, Beliveau R (2006) The Survivin-mediated radioresistant phenotype of glioblastomas is regulated by RhoA and inhibited by the green tea polyphenol (-)-epigallocatechin-3-gallate. Brain Res 1071(1):1–9. doi:10.1016/j.brainres.2005.10.009
Altieri DC (2001) The molecular basis and potential role of survivin in cancer diagnosis and therapy. Trends Mol Med 7(12):542–547. doi:10.1016/S1471-4914(01)02243-2
Kim EJ, Choi CH, Park JY, Kang SK, Kim YK (2008) Underlying mechanism of quercetin-induced cell death in human glioma cells. Neurochem Res 33:971–979
Kang DW, Choi CH, Park JY, Kang SK, Kim YK (2008) Ciglitazone induces caspase-independent apoptosis through down-regulation of XIAP and survivin in human glioma cells. Neurochem Res 33(3):551–561. doi:10.1007/s11064-007-9475-x
Ai Z, Yin L, Zhou X, Zhu Y, Zhu D, Yu Y, Feng Y (2006) Inhibition of survivin reduces cell proliferation and induces apoptosis in human endometrial cancer. Cancer 107(4):746–756. doi:10.1002/cncr.22044
Yao LL, Wang YG, Cai WJ, Yao T, Zhu YC (2007) Survivin mediates the anti-apoptotic effect of delta-opioid receptor stimulation in cardiomyocytes. J Cell Sci 120(Pt 5):895–907. doi:10.1242/jcs.03393
Mitchell C, Park MA, Zhang G, Yacoub A, Curiel DT, Fisher PB, Roberts JD, Grant S, Dent P (2007) Extrinsic pathway- and cathepsin-dependent induction of mitochondrial dysfunction are essential for synergistic flavopiridol and vorinostat lethality in breast cancer cells. Mol Cancer Ther 6(12 Pt 1):3101–3112. doi:10.1158/1535-7163.MCT-07-0561
Martindale JL, Holbrook NJ (2002) Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol 192:1–15
Yang HY, Kim J, Chung GH, Lee JC, Jang YS (2007) Cross-linking of MHC class II molecules interferes with phorbol 12, 13-dibutyrate-induced differentiation of resting B cells by inhibiting Rac-associated ROS-dependent ERK/p38 MAP kinase pathways leading to NF-kappaB activation. Mol Immunol 44(7):1577–1586. doi:10.1016/j.molimm.2006.08.017
Su YT, Chang HL, Shyue SK, Hsu SL (2005) Emodin induces apoptosis in human lung adenocarcinoma cells through a reactive oxygen species-dependent mitochondrial signaling pathway. Biochem Pharmacol 70(2):229–241. doi:10.1016/j.bcp.2005.04.026
Rahmani M, Reese E, Dai Y, Bauer C, Payne SG, Dent P, Spiegel S, Grant S (2005) Coadministration of histone deacetylase inhibitors and perifosine synergistically induces apoptosis in human leukemia cells through Akt and ERK1/2 inactivation and the generation of ceramide and reactive oxygen species. Cancer Res 65(6):2422–2432. doi:10.1158/0008-5472.CAN-04-2440
Lee WC, Choi CH, Cha SH, Oh HL, Kim YK (2005) Role of ERK in hydrogen peroxide-induced cell death of human glioma cells. Neurochem Res 30:263–270
Park BG, Yoo CI, Kim HT, Kwon CH, Kim YK (2005) Role of mitogen-activated protein kinases in hydrogen peroxide-induced cell death in osteoblastic cells. Toxicology 215(1–2):115–125. doi:10.1016/j.tox.2005.07.003
Russo M, Palumbo R, Tedesco I, Mazzarella G, Russo P, Iacomino G, Russo GL (1999) Quercetin and anti-CD95(Fas/Apo1) enhance apoptosis in HPB-ALL cell line. FEBS Lett 462(3):322–328. doi:10.1016/S0014-5793(99)01544-6
Acknowledgement
This work was supported by the MRC program of MOST/KOSEF (R13-2005-009) in Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeong, J.C., Kim, M.S., Kim, T.H. et al. Kaempferol Induces Cell Death Through ERK and Akt-Dependent Down-Regulation of XIAP and Survivin in Human Glioma Cells. Neurochem Res 34, 991–1001 (2009). https://doi.org/10.1007/s11064-008-9868-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-008-9868-5