Summary
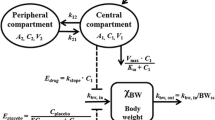
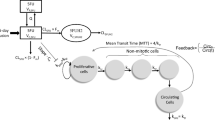
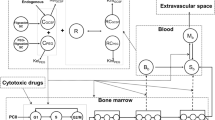
Aim To investigate the potential of a model for chemotherapy-induced myelosuppression to predict the full time-course of myelosuppression in patients based on rat data. Methods White blood cell counts were determined in rats after administration of 5-fluorouracil, epirubicin, cyclophosphamide, docetaxel, paclitaxel or etoposide. Pharmacokinetic models were used to predict the concentration-time profile in each rat. A semi-physiological model of myelosuppression was applied to the rat data. The drug-related parameter Slope was allowed to differ between drugs. The analysis was performed in NONMEM VI. Time-courses of myelosuppression in patients were predicted for each drug based on patient pharmacokinetic models, typical system-related parameters previously determined in patients and the rat Slope estimates in the present study. Results The semi-physiological model of myelosuppression fit the rat data well and the estimated maturation time in rats (53 h) was approximately half of the previous estimate in patients. The relative difference in Slope estimates for rats and patients based on total drug concentrations ranged between 28% to 8-fold for the six drugs. The differences reduced to 8–37% for all drugs when correcting the rat Slope estimates for species difference in protein binding and in CFU-GM assay sensitivity. Conclusions This method for interspecies scaling was successful in predicting the time-course of myelosuppression in patients based on rat data. Predictions improved when species differences in protein binding and CFU-GM assay sensitivity were accounted for. The approach appears promising for predicting myelosuppression in patients early in development.




Similar content being viewed by others
References
Friberg LE, Henningsson A, Maas H, Nguyen L, Karlsson MO (2002) Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol 20:4713–4721
Sandstrom M, Lindman H, Nygren P, Lidbrink E, Bergh J, Karlsson MO (2005) Model describing the relationship between pharmacokinetics and hematologic toxicity of the epirubicin-docetaxel regimen in breast cancer patients. J Clin Oncol 23:413–421
Sandstrom M, Lindman H, Nygren P, Johansson M, Bergh J, Karlsson MO (2006) Population analysis of the pharmacokinetics and the haematological toxicity of the fluorouracil-epirubicin-cyclophosphamide regimen in breast cancer patients. Cancer Chemother Pharmacol 58:143–156
Troconiz IF, Garrido MJ, Segura C, Cendros JM, Principe P, Peraire C, Obach R (2006) Phase I dose-finding study and a pharmacokinetic/pharmacodynamic analysis of the neutropenic response of intravenous diflomotecan in patients with advanced malignant tumours. Cancer Chemother Pharmacol 57:727–735. doi:10.1007/s00280-005-0112-6
Latz JE, Karlsson MO, Rusthoven JJ, Ghosh A, Johnson RD (2006) A semimechanistic-physiologic population pharmacokinetic/pharmacodynamic model for neutropenia following pemetrexed therapy. Cancer Chemother Pharmacol 57:412–426
Leger F, Loos WJ, Bugat R, Mathijssen RH, Goffinet M, Verweij J, Sparreboom A, Chatelut E (2004) Mechanism-based models for topotecan-induced neutropenia. Clin Pharmacol Ther 76:567–578
Zandvliet AS, Siegel-Lakhai WS, Beijnen JH, Copalu W, Etienne-Grimaldi MC, Milano G, Schellens JH, Huitema AD (2008) PK/PD model of indisulam and capecitabine: interaction causes excessive myelosuppression. Clin Pharmacol Ther 83:829–839. doi:6100344 [pii] 10.1038/sj.clpt.6100344
van Kesteren C, Zandvliet AS, Karlsson MO, Mathot RA, Punt CJ, Armand JP, Raymond E, Huitema AD, Dittrich C, Dumez H, Roche HH, Droz JP, Ravic M, Yule SM, Wanders J, Beijnen JH, Fumoleau P, Schellens JH (2005) Semi-physiological model describing the hematological toxicity of the anti-cancer agent indisulam. Invest New Drugs 23:225–234. doi:10.1007/s10637-005-6730-3
Brain EG, Rezai K, Lokiec F, Gutierrez M, Urien S (2008) Population pharmacokinetics and exploratory pharmacodynamics of ifosfamide according to continuous or short infusion schedules: an n = 1 randomized study. Br J Clin Pharmacol 65:607–610. doi:BCP3095 [pii] 10.1111/j.1365-2125.2007.03095.x
Kloft C, Wallin J, Henningsson A, Chatelut E, Karlsson MO (2006) Population pharmacokinetic-pharmacodynamic model for neutropenia with patient subgroup identification: comparison across anticancer drugs. Clin Cancer Res 12:5481–5490
Mordenti J (1986) Man versus beast: pharmacokinetic scaling in mammals. J Pharm Sci 75:1028–1040
Ulich TR, del Castillo J (1991) The hematopoietic and mature blood cells of the rat: their morphology and the kinetics of circulating leukocytes in control rats. Exp Hematol 19:639–648
Dancey JT, Deubelbeiss KA, Harker LA, Finch CA (1976) Neutrophil kinetics in man. J Clin Invest 58:705–715
Newell DR, Burtles SS, Fox BW, Jodrell DI, Connors TA (1999) Evaluation of rodent-only toxicology for early clinical trials with novel cancer therapeutics. Br J Cancer 81:760–768
Woo S, Jusko WJ (2007) Interspecies comparisons of pharmacokinetics and pharmacodynamics of recombinant human erythropoietin. Drug Metab Dispos 35:1672–1678
Zuideveld KP, Van der Graaf PH, Peletier LA, Danhof M (2007) Allometric scaling of pharmacodynamic responses: application to 5-Ht1A receptor mediated responses from rat to man. Pharm Res 24:2031–2039
Yassen A, Olofsen E, Kan J, Dahan A, Danhof M (2007) Animal-to-human extrapolation of the pharmacokinetic and pharmacodynamic properties of buprenorphine. Clin Pharmacokinet 46:433–447
Lepist EI, Jusko WJ (2004) Modeling and allometric scaling of s(+)-ketoprofen pharmacokinetics and pharmacodynamics: a retrospective analysis. J Vet Pharmacol Ther 27:211–218
Gronert GA, Fung DL, Jones JH, Shafer SL, Hildebrand SV, Disbrow EA (1995) Allometry of pharmacokinetics and pharmacodynamics of the muscle relaxant metocurine in mammals. Am J Physiol 268:R85–91
Pessina A, Albella B, Bueren J, Brantom P, Casati S, Gribaldo L, Croera C, Gagliardi G, Foti P, Parchment R, Parent-Massin D, Sibiril Y, Van Den Heuvel R (2001) Prevalidation of a model for predicting acute neutropenia by colony forming unit granulocyte/macrophage (CFU-GM) assay. Toxicol In Vitro 15:729–740
Simonsen LE, Wahlby U, Sandstrom M, Freijs A, Karlsson MO (2000) Haematological toxicity following different dosing schedules of 5- fluorouracil and epirubicin in rats. Anticancer Res 20:1519–1525
Friberg LE, Freijs A, Sandström M, Karlsson MO (2000) Semiphysiological model for the time course of leukocytes after varying schedules of 5-fluorouracil in rats. J Pharmacol Exp Ther 295:734–740
Sandström M, Simonsen LE, Freijs A, Karlsson MO (1999) The pharmacokinetics of epirubicin and docetaxel in combination in rats. Cancer Chemother Pharmacol 44:469–474
Jonsson E, Friberg LE, Karlsson MO, Hassan SB, Freijs A, Hansen K, Larsson R (2000) Determination of drug effect on tumour cells, host animal toxicity and drug pharmacokinetics in a hollow-fibre model in rats. Cancer Chemother Pharmacol 46:493–500
Burgio DE, Gosland MP, McNamara aPJ (1998) Effects of P-glycoprotein modulators on etoposide elimination and central nervous system distribution. J Pharmacol Exp Ther 287:911–917
Beal SL, Sheiner LB, Boeckmann AJ (eds) (1989–2006) NONMEM Users Guides Icon Development Solutions, Ellicott City, Maryland, USA
Wilkins J, Karlsson MO, Jonsson EN (2006) Patterns and power for the visual predictive check. In: PAGE 15ed., ppAbstr 1028. www.page-meeting.org/?abstract=1029
Machiavelli M, Leone BA, Romero A, Rabinovich MG, Vallejo CT, Bianco A, Perez JE, Rodriguez R, Cuevas MA, Alvarez LA et al (1991) Advanced colorectal carcinoma. A prospective randomized trial of sequential methotrexate, 5-fluorouracil, and leucovorin versus 5-fluorouracil alone. Am J Clin Oncol 14:211–217
Woolley PV, Ayoob MJ, Smith FP, Lokey JL, DeGreen P, Marantz A, Schein PS (1985) A controlled trial of the effect of 4-hydroxypyrazolopyrimidine (allopurinol) on the toxicity of a single bolus dose of 5-fluorouracil. J Clin Oncol 3:103–109
Hassan SB, Haglund C, Aleskog A, Larsson R, Lindhagen E (2007) Primary lymphocytes as predictors for species differences in cytotoxic drug sensitivity. Toxicol In Vitro 21:1174–1181
Celio LA, DiGregorio GJ, Ruch E, Pace JN, Piraino AJ (1983) 5-Fluorouracil concentrations in rat plasma, parotid saliva, and bile and protein binding in rat plasma. J Pharm Sci 72:597–599
Garrett ER, Hurst GH, Green JR Jr (1977) Kinetics and mechanisms of drug action of microorganisms XXIII: microbial kinetic assay for fluorouracil in biological fluids and its application to human pharmacokinetics. J Pharm Sci 66:1422–1429
Hall KS, Endresen L, Schjerven L, Rugstad HE (1990) The influence of partial hepatectomy on the pharmacokinetics of preoperatively injected 4′-epidoxorubicin in rats. Cancer Chemother Pharmacol 26:444–448
Robert J, Gianni L (1993) Pharmacokinetics and metabolism of anthracyclines. Cancer Surv 17:219–252
Voelcker G, Wagner T, Hohorst HJ (1976) Identification and pharmacokinetics of cyclophosphamide (NSC-26271) metabolites in vivo. Cancer Treat Rep 60:415–422
Lu H, Chan KK (2006) Pharmacokinetics of N-2-chloroethylaziridine, a volatile cytotoxic metabolite of cyclophosphamide, in the rat. Cancer Chemother Pharmacol 58:532–539
Moore MJ (1991) Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet 20:194–208
Bissery MC, Nohynek G, Sanderink GJ, Lavelle F (1995) Docetaxel (Taxotere): a review of preclinical and clinical experience. Part I: Preclinical experience. Anticancer Drugs 6:339–355, 363–338
Urien S, Barre J, Morin C, Paccaly A, Montay G, Tillement JP (1996) Docetaxel serum protein binding with high affinity to alpha 1-acid glycoprotein. Invest New Drugs 14:147–151
Henningsson A, Karlsson MO, Vigano L, Gianni L, Verweij J, Sparreboom A (2001) Mechanism-based pharmacokinetic model for paclitaxel. J Clin Oncol 19:4065–4073
Brouwer E, Verweij J, De Bruijn P, Loos WJ, Pillay M, Buijs D, Sparreboom A (2000) Measurement of fraction unbound paclitaxel in human plasma. Drug Metab Dispos 28:1141–1145
Fleming RA, Arbuck SG, Stewart CF (1991) Interspecies differences in in vitro etoposide plasma protein binding. Biochem Pharmacol 42:2246–2249
Pessina A, Malerba I, Gribaldo L (2005) Hematotoxicity testing by cell clonogenic assay in drug development and preclinical trials. Curr Pharm Des 11:1055–1065
Masubuchi N, May RD, Atsumi R (2004) A predictive model of human myelotoxicity using five camptothecin derivatives and the in vitro colony-forming unit granulocyte/macrophage assay. Clin Cancer Res 10:6722–6731. doi:10/19/6722 [pii] 10.1158/1078-0432.CCR-04-0721
Houston JB, Carlile DJ (1997) Prediction of hepatic clearance from microsomes, hepatocytes, and liver slices. Drug Metab Rev 29:891–922. doi:10.3109/03602539709002237
Segura C, Bandres E, Troconiz IF, Garcia-Foncillas J, Sayar O, Dios-Vieitez C, Renedo MJ, Garrido MJ (2004) Hematological response of topotecan in tumor-bearing rats: modeling of the time course of different cellular populations. Pharm Res 21:567–573
Acknowledgement
We would like to thank Britt Jansson and Jessica Strömgren for excellent technical assistance. The study was supported by the Swedish Cancer Society. Lena Friberg was during part of this work supported by the Knut and Alice Wallenberg foundation, Sweden.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Friberg, L.E., Sandström, M. & Karlsson, M.O. Scaling the time-course of myelosuppression from rats to patients with a semi-physiological model. Invest New Drugs 28, 744–753 (2010). https://doi.org/10.1007/s10637-009-9308-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-009-9308-7