Abstract
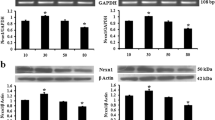
(1) Presenilin (PS) expression is regulated by several cellular and extracellular factors which change with age and sex. Both age and sex are key risk factors for Alzheimer’s disease (AD), which is linked to mutations in PS genes. (2) We have analyzed the effect of age and sex on PS expression by northern hybridization and western blot analysis using the cerebral cortex of adult (24 ± 2 weeks) and old (65 ± 5 weeks) mice. (3) Our results demonstrate that PS1 was downregulated and PS 2 was upregulated in old mice of both sexes. The level of PS 1 was relatively higher and that of PS 2 was lower in female than male mice of same age group. Taken together, these findings show age and sex dependent alteration in PS expression, which in turn may influence the signal transduction pathways and consequently brain functions.


Similar content being viewed by others
References
Bazan NG, Lukiw WJ (2002) Cyclooxygenase-2 and presenilin-1 gene expression induced by interleukin-1 beta and amyloid beta 42 peptide is potentiated by hypoxia in primary human neural cells. J Biol Chem 277:30359–30367
Bertoni-Freddari C, Giuli C, Pieri C, Paci D (1986) Age-related morphological rearrangements of synaptic junctions in the rat cerebellum and hippocampus. Arch Gerontol Geriatr 5:297–304
Capell A, Grunberg J, Pesold B, Diehlmann A, Citron M, Nixon R, Beyreuther K, Selkoe DJ, Haass C (1998) The proteolytic fragments of the Alzheimer’s disease-associated presenilin 1 form heterodimers and occurs as a 100–150 kDa molecular mass complex. J Biol Chem 273:3205–3211
Counts SE, Lah JJ, Levey AI (2001) The regulation of presenilin 1 by nerve growth factor. J Neurochem 76:679–689
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Da Costa CA, Mattson MP, Ancolio K, Checler F (2003) The C-terminal fragment of presenilin-2 triggers p53-mediated staurosporine-induced apoptosis, a function independent of the presenilinase-derived N-terminal counterpart. J Biol Chem 278:12064–12069
De Strooper B, Beullens M, Contreras B, Levesque L, Craessaerts K, Cordell B, Moechars D, Bollen M, Fraser P, St. George-Hyslop P, Leuven FV (1997) Phosphorylation, subcellular localization, and membrane orientation of the Alzhermer’s disease-associated presenilins. J Biol Chem 272:3590–3598
Fluhrer R, Friedlein A, Haass C, Walter J (2004) Phosphorylation of presenilin1 at the caspase recognition site regulates its proteolytic processing and the progression of apoptosis. J Biol Chem 279:1585–1593
Ghosh S, Thakur MK (2007a) PS1 Expression is downregulated by gonadal steroids in adult mouse brain. Neurochem Res. doi:10.1007/s11064-007-9424-8
Ghosh S, Thakur MK (2007b) PS2 protein expression is upregulated by sex steroids in the cerebral cortex of aging mice. Neurochem Res. doi:10.1016/j.neuint. 2007.07.015
Hashimoto-Gotoh T, Tsujimura A, Watanabe Y, Iwabe N, Miyata T, Tabira T (2003) A unifying model for functional difference and redundancy of presenilin-1 and -2 in cell apoptosis and differentiation. Gene 323:115–123
Higashide S, Morikawa K, Okumura M, Kondo S, Ogata M, Murakami T, Yamashita A, Kanemoto S, Manabe T, Imaizumi K (2004) Identification of cis-acting element for alternative splicing of presenilin 2 exon 5 under hypoxic stress conditions. J Neurochem 91:1191–1198
Iwatsubo T (2004). The γ-secretase complex: machinery for intramembrane proteolysis. Curr Opin Neurobiol 14:379–383
Kern A, Roempp B, Prager K, Walter J, Behl C (2006) Down-regulation of endogenous amyloid precursor protein processing due to cellular aging. J Biol Chem 281:2405–2457
Kimura N, Nakamura S-I, Honda T, Takashima A, Nakayama H, Ono F, Sakakibara I, Doi K, Kawamura S, Yoshikawa Y (2001) Age related changes in the localization of presenilin-1 in cynomolgus monkey brain. Brain Res 922:30–41
Kovacs DM, Fausett HJ, Page KJ, Kim T-W, Mori RD (1996) Alzheimer associated presenilins 1 and 2: neuronal expression in brain and localization to intracellular membranes in mammalian cells. Nat Med 2:224–229
Lee MK, Slunt HH, Martin LJ, Thinakaran G, Kim G, Gandy SE, Seeger M, Koo E, Price DL, Sisodia SS (1996). Expression of presenilin 1 and 2 (PS1 and PS2) in human and murine tissues. J Neurosci 16:7513–7525
Levy-Lahad E, Wijsman EM, Nemens E, Anderson L, Goddard KAB, Weber JL, Bird TD, Schellenberg GD (1995) A familial Alzheimer’s disease locus on chromosome1. Science 269:970–973
Lukiw WJ, Gordon WC, Rogaev EI, Thompson H, Bazan NG (2001) Presenilin-2 (PS2) expression up-regulation in a model of retinopathy of prematurity and pathoangiogenesis. Neuroreport 12:53–57
Massey LK, Mah AL, Ford DL, Miller J, Liang J, Doong H, Monteiro MJ (2004) Overexpression of ubiquilin decreases ubiquitination and degradation of presenilin proteins. J Alzheimers Dis 6:79–92
Mitsuda N, Ohkubo N, Tamatani M, Lee Y, Taniguchi M, Namikawa K, Kiyama H, Yamaguchi A, Sato N, Sakata K, Ogihara T, Vitek MP, Tohyama M (2001) Activated cAMP-response element-binding protein regulates neuronal expression of presenilin 1. J Biol Chem 276:9688–9698
Nixon RA (2003) The calpains in aging and age related diseases. Aging Res Rev 2:407–418
Parks AL, Curtis D (2007) Presenilin diversifies its portfolio. Trends Genet 23:140–150
Ren RF, Lah JJ, Diehlmann A, Kim ES, Hawver DB, Levey AI, Beyreuther K, Flanders KC (1999) Differential effects of transforming growth factor-beta (s) and glial-cell line derived neurotrophic factor on gene expression of presenilin-1 in human post-mitotic neurons and astrocytes. Neuroscience 93:1041–1049
Renbaum P, Beeri R, Gabai E, Amiel M, Gal M, Ehrengruber MU, Levy-Lahad E (2003) Egr-1 upregulates the Alzheiemr’s disease presenilin-2 gene in neuronal cells. Gene 318:113–124
Ribaut-Barassin C, Dupont JL, Haeberle AM, Bombarde G, Huber G, Moussaoui S, Mariani J, Bailly Y (2003) Alzheimer’s disease proteins in cerebellar and hippocampal synapses during postnatal development and aging of the rat. Neuroscience 120:405–423
Satoh J, Kuroda Y (1999) Constitutive and cytokine—regulated expression of presenilin-1 and presenilin-2 genes in human neural cell lines. Neuropathol Appl Neurobiol 25:492–503
Saura CA, Choi S, Beglopoulos V, Malkani S, Zhang D, Rao BSS, Chattarji S, Kelleher RL, Kandel ER, Duff K, Kirkwood A, Shen J (2004) Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron 42:23–36
Scheibel A, Conrad T, Perdue S, Tomiyasu U, Wechsler A (1990) A quantitative study of dendrite complexity in selected areas of the human cerebral cortex. Brain Cognition 12:85–101
Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, Tsuda T, Mar L, Foncin JF, Bruni AC, Montesi MP, Sorbi S, Rainero I, Pinessi L, Nee L, Chumakov I, Pollen D, Brookes A, Sanseau P, Polinsky RJ, Wasco W, Da Silva HAR, Haines JL, Pericak-Vance MA, Tanzi RE, Roses AD, Fraser PE, Rommens JM, St. George-Hyslop PH (1995) Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 375:754–760
Silhot M, Bonnichon V, Rage F, Tapia-Arancibia L (2005) Age related changes in brain derived neurotrophic factor and tyrosine kinase receptor isoforms in the hippocampus and hypothalamus in male rats. Neuroscience 132:613–624
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985). Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Thinakaran G, Borchelt DR, Lee MK, Slunt HH, Spitzer L, Kim G, Ratovitski T, Davenport F, Nordstedt C, Seeger M, Hardy J, Levey AI, Gandy S, Jenkins NA, Copeland NG, Price DL, Sisodia SS (1996). Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron 17:181–190
Tokuhiro S, Tomita T, Iwata H, Kosaka T, Saido TC, Maruyama K, Iwatsubo T (1998) The presenilin 1 mutation (M146 V) linked to familial Alzheimer disease attenuates the neuronal differentiation of NTera 2 cells. Biochem Biophys Res Commun 244:751–755
Walter J, Schindzielorz A, Grunberg J, Haass C (1999). Phosphorylation of presenilin 2 regulates its cleavage by caspases and retards progression of apoptosis. Proc Natl Acad Sci USA 96:1391–1396
Williams B, Granholm AC, Sambamurti K (2007) Age dependent loss of NGF signaling in the rat basal forebrain is due to disrupted MAPK activation. Neurosci Lett 413:110–114
Acknowledgments
We thank Bart de Strooper (Belgium) for the generous gift of PS1 and PS2 cDNA constructs, Sam Gandy (Philadelphia, USA) for PS1 antiserum, and Helmut Jacobsen (Basel, Switzerland) for PS2 antiserum. This work was supported by a research fellowship from the University Grants commission to SG, and grants from the Department of Biotechnology (P-07/311/2004) and Department of Science and Technology (P-07/317/2005), Government of India, to MKT.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thakur, M.K., Ghosh, S. Age and Sex Dependent Alteration in Presenilin Expression in Mouse Cerebral Cortex. Cell Mol Neurobiol 27, 1059–1067 (2007). https://doi.org/10.1007/s10571-007-9214-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-007-9214-5