Abstract
Background
Diabetic retinopathy (DR) is one of the leading causes of blindness, affecting over 90% of diabetics. Exendin-4 (E4) is a potent and long-acting agonist of the glucagon-like peptide-1 (GLP-1) receptor. GLP-1 is an insulinotropic gut peptide, which normalizes blood glucose level and is now being tested in clinical trials as a treatment for diabetes. The purpose of our study was to explore the protective effect of subcutaneous (sc.) exendin-4 analogue (E4a) on early DR.
Methods
Expression of GLP-1R was detected at both mRNA and protein levels and verified by immunohistochemistry. Thirty-six Sprague–Dawley (SD) rats were included in the experiment. Diabetes was induced by intraperitoneal injection (ip) of streptozotocin (STZ). The rats were divided into three groups: normal control (N), diabetic control (D) and E4a-treated diabetic (E4a) group. For the E4a group, the rats were treated with E4a (sc.0.05 μg/g BW/day); for the N and D groups, the rats were treated with normal saline (NS, sc). Blood glucose levels and body weight were measured weekly. Electroretinogram (ERG) was performed 1 and 3 months after diabetes onset. The retinal thickness and cell counts in each layer were evaluated under light microscopy after ERG examination.
Results
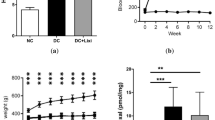
GLP-1R was expressed at both mRNA and protein levels in the retina of SD rats. Immunostaining of the rat retina revealed that GLP-1R was predominantly expressed in the inner layer of the retina. E4a can reduce the blood glucose level of diabetic rats to the normal control level. B-wave amplitudes and OPs decreased with the progress of diabetes, and E4a prevents the loss of b-wave amplitude and OPs caused by diabetes. The retinal thickness was reduced in a diabetes-duration-dependent fashion. The cell counts of both ONL and INL were reduced accordingly in the diabetic rats. E4a prevented cell loss and maintained a normal thickness.
Conclusions
GLP-1R is expressed in rat retina. Apoptosis is an important constituent of retinal cell death in early DR. E4a administration can reverse the changes of ERG, prevent the retinal cell death and maintain normal retinal thickness in diabetic rats. Therefore, this is a potent approach for treatment of early DR.




Similar content being viewed by others
References
Abraham EJ, Leech CA, Lin JC, Zulewski H, Habener JF (2002) Insulinotropic hormone glucagon-like peptide-1 differentiation of human pancreatic islet-derived progenitor cells into insulin-producing cells. Endocrinology 143:3152–3161
Antonetti DABA, Bronson SK, Freeman WM, Gardner TW, Jefferson LS, Kester M, Kimball SR, Krady JK, LaNoue KF, Norbury CC, Quinn PG, Sandirasegarane L, Simpson IA, JDRF Diabetic Retinopathy Center Group (2006) Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes 55:2401–2411
Barber AJ (2003) A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry 27:283–290
Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM (2005) Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes 54:146–151
Bresnick GH, Palta M (1987) Oscillatory potential amplitudes. Relation to severity of diabetic retinopathy. Arch Ophthalmol 105:929–933
Buteau J, Roduit R, Susini S, Prentki M (1999) Glucagon-like peptide-1 promotes DNA synthesis, activates phosphatidylinositol 3-kinase and increases transcription factor pancreatic and duodenal homeobox gene 1 (PDX-1) DNA binding activity in beta (INS-1)-cells. Diabetologia 42:856–864
Drucker DJ (2001) Minireview: the glucagon-like peptides. Endocrinology 142:521–527
Dupre J (2005) Glycaemic effects of incretins in Type 1 diabetes mellitus: a concise review, with emphasis on studies in humans. Regul Pept 128:149–157
Dupre J, Behme MT, McDonald TJ (2004) Exendin-4 normalized postcibal glycemic excursions in Type 1 diabetes. J Clin Endocrinol Metab 89:3469–3473
During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, Haile CN (2003) Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med 9:1173–1179
Fletcher EL, Phipps JA, Ward MM, Puthussery T, Wilkinson-Berka JL (2007) Neuronal and glial cell abnormality as predictors of progression of diabetic retinopathy. Curr Pharm Des 13:2699–2712
Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW (2002) Diabetic retinopathy: more than meets the eye. Surv Ophthalmol 47(Suppl 2):S253–262
Gershengorn MC, Hardikar AA, Wei C, Geras-Raaka E, Marcus-Samuels B, Raaka BM (2004) Epithelial-to-mesenchymal transition generates proliferative human islet precursor cells. Science 306:2261–2264
Goke R, Linn T, Schmidt H, Krause M, Eng J et al (1993) Exendin-4 is a high potency agonist and truncated exendin-(9–39)-amide an antagonist at the glucagon-like peptide 1-(7–36)-amide receptor of insulin-secreting beta-cells. J Biol Chem 268:19650–19655
Greig NHHH, De Ore KA, Jani D, Wang Y, Zhou J et al (1999) Once daily injection of exendin-4 to diabetic mice achieves long-term beneficial effects on blood glucose concentrations. Diabetologia 42:45–50
Guerci B, Martin CS (2008) Exenatide: its position in the treatment of type 2 diabetes. Ann Endocrinol (Paris) 69:201–209
Hancock HA, Kraft TW (2004) Oscillatory potential analysis and ERGs of normal and diabetic rats. Invest Ophthalmol Vis Sci 45:1002–1008
Harkavyi A, Abuirmeileh A, Lever R, Kingsbury AE, Biggs CS, Whitton PS (2008) Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease. J Neuroinflammation 5:19
Holopigian K, Greenstein VC, Seiple W, Hood DC, Ritch R (2000) Electrophysiologic assessment of photoreceptor function in patients with primary open-angle glaucoma. J Glaucoma 9:163–168
Holst JJ (2002) Therapy of type 2 diabetes mellitus based on the actions of glucagon-like peptide-1. Diabetes Metab Res Rev 18:430–441
James F, List HH, Habener JF (2006) Glucagon-like peptide-1 receptor and proglucagon expression in mouse skin. Regulatory Peptides 134:149–157
Juen S, Kieselbach GF (1990) Electrophysiological changes in juvenile diabetics without retinopathy. Arch Ophthalmol 108:372–375
Kieffer TJ, Francis Habener J (1999) The Glucagon-like peptides. Endocr Rev 20:876–913
Layton CJ, Safa R, Osborne NN (2007) Oscillatory potentials and the b-Wave: partial masking and interdependence in dark adaptation and diabetes in the rat. Graefes Arch Clin Exp Ophthalmol 245:1335–1345
Lupi R, Mancarella R, Del Guerra S, Bugliani M, Del Prato S, Boggi U, Mosca F, Filipponi F, Marchetti P (2008) Effects of exendin-4 on islets from type 2 diabetes patients. Diabetes Obes Metab 10:515–519
Madsen-Bouterse SA, Kowluru RA (2008) Oxidative stress and diabetic retinopathy: Pathophysiological mechanisms and treatment perspectives. Rev Endocr Metab Disord 9:315–327
Malendowicz LK, Macchi C, Nussdorfer GG, Nowak KW, Zyterska A, Ziolkowska A (2003) Effects of prolonged exendin-4 administration on entero-insular axis of normal and streptozotocin-induced diabetic rats. Int J Mol Med 11:763–766
Malendowicz LK, Neri G, Nussdorfer GG, Nowak KW, Zyterska A, Ziolkowska A (2003) Prolonged exendin-4 administration stimulates pituitary-adrenocortical axis of normal and streptozotocin-induced diabetic rats. Int J Mol Med 12:593–596
Marmor MF, Holder GE, Seeliger MW, Yamamoto S (2004) Standard for clinical electroretinography (2004 update). Doc Ophthalmol 108:107–114
Nauck MAKN, Ørskov C, Holst JJ, Willms B, Creutzfeldt W (1993) Normalization of fasting hyperglycemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 36:741–744
P Schlatter CB, Drewe J, Gutmann H (2007) Glucagon-like peptide 1 receptor expression in primary porcine proximal tubular cells. Regulatory Peptides 141:120–128
Pradeepa R, Anitha B, Mohan V, Ganesan A, Rema M (2008) Risk factors for diabetic retinopathy in a South Indian Type 2 diabetic population—the Chennai Urban Rural Epidemiology Study (CURES) Eye Study 4. Diabet Med 25:536–542
Premalatha GVK, Deepa R et al (2002) Prevalence of non-diabetic renal disease in type 2 diabetic patients in a diabetes centre in Southern India. J Assoc Physicians India 50:1135–1139
Sakai H, Tani Y, Shirasawa E, Shirao Y, Kawasaki K (1995) Development of electroretinographic alterations in streptozotocin-induced diabetes in rats. Ophthalmic Res 27:57–63
Strauss A, Moskalenko V, Chodnevskaja I, Timm S, Thiede A, Otto C, Ulrichs K (2008) Exendin-4 improves the oral glucose tolerance in diabetic rats: pancreas regeneration, better function of pancreatic islets, or impaired glucose uptake? Transplant Proc 40:533–535
Sundling V, Gulbrandsen P, Jervell J, Straand J (2008) Care of vision and ocular health in diabetic members of a national diabetes organization: a cross-sectional study. BMC Health Serv Res 8:159
Szayna M, Doyle ME, Betkey JA, Holloway HW, Spencer RGS, Greig NH, Egan JM (2000) Exendin-4 decelerates food intake, weight gain, and fat deposition in Zucker rats. Endocrinology 141:1936–1941
Vadala M, Anastasi M, Lodato G, Cillino S (2002) Electroretinographic oscillatory potentials in insulin-dependent diabetes patients: A long-term follow-up. Acta Ophthalmol Scand 80:305–309
Wong TY, Cheung N, Tay WT, Wang JJ, Aung T, Saw SM, Lim SC, Tai ES, Mitchell P (2008) Prevalence and Risk Factors for Diabetic Retinopathy. The Singapore Malay Eye Study. Ophthalmology [June 25, Epub ahead of print]
Xu G, Kaneto H, Laybutt DR, Duvivier-Kali VF, Trivedi N, Suzuma K, King GL, Weir GC, Bonner-Weir S (2007) Downregulation of GLP-1 and GIP receptor expression by hyperglycemia: possible contribution to impaired incretin effects in diabetes. Diabetes 56:1551–1558
Zhang J, Wu Y, Jin Y, Ji F, Sinclair SH, Luo Y, Xu G, Lu L, Dai W, Yanoff M, Li W, Xu G-T (2008) Intravitreal injection of erythropoietin protects both retinal vascular and neuronal cells in early diabetes. Invest Ophthalmol Vis Sci 49:732–742
Author information
Authors and Affiliations
Corresponding authors
Additional information
Wen Ye and Guo-Tong Xu are co-corresponding authors who contributed equally to this study.
Rights and permissions
About this article
Cite this article
Zhang, Y., Wang, Q., Zhang, J. et al. Protection of exendin-4 analogue in early experimental diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 247, 699–706 (2009). https://doi.org/10.1007/s00417-008-1004-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-008-1004-3