Abstract
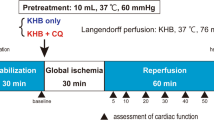
The present study investigated the tolerance of the isolated rat heart to ischemia–reperfusion after administration of trimetazidine (TMZ) at different experimental phases, as well as the possible involvement of p38 MAPK and JNKs in this response. Isolated rat hearts were perfused in Langendorff mode. Untreated hearts after stabilization (S) were subjected to 20 min of zero-flow global ischemia (I) and 45 min of reperfusion (R), (NORM), n = 9. TMZ (10–5 M) was administered (in the perfusate): a) only at S phase, (TMZ–STAB), n = 8, b) only at R, (TMZ–REP), n = 8 and c) during both S and R, (TMZ–STAB+REP), n = 8. Recovery of left ventricular developed pressure at 45 min of R (Rec) was significantly higher in TMZ–STAB and TMZ–STAB+REP and LDH release was lower in TMZ–STAB+REP and TMZ–STAB than NORM, [1153.2 (121.0) and 1152.1 (86.8) vs 1573.5 (138.2), P < 0.05]. TMZ induced cardioprotection did not involve p38 MAPK and JNKs. Phospho–p38 MAPK and JNKs levels after I/R were not changed with TMZ treatment. In TMZ–REP, Rec and LDH release were similar to NORM, but the rate of functional recovery (ratio of Rec at 10 min of R to Rec) was 86.7% (13.3) for TMZ–REP vs 53.8% (7.7) for NORM, P < 0.05. This effect was associated with decreased myocardial lactate content early at reperfusion. In conclusion, preischemic administration of TMZ protects against I/R injury while TMZ given only at reperfusion accelerates recovery of function without reducing the extent of injury.
Similar content being viewed by others
References
Allibardi S, Chierchia SL, Margonato V, Merati G, Neri G, Dell’Antonio G, Samaja M (1998) Effects of trimetazidine on metabolic and functional recovery of postischaemic rat hearts. Cardiovasc Drugs Ther 12:543–549
Armstrong SC (2004) Protein kinase activation and myocardial ischemia/reperfusion injury. Cardiovasc Res 61:427–436
Bogoyevitch M, Gillespie-Brown J, Ketterman A, Fuller SJ, Ben-Levy R, Ashworth A, Marshall CJ, Sugden PH (1996) Stimulation of the stress-activated mitogen-activated protein kinase subfamilies in perfused heart: p38/RK mitogen-activated protein kinases and c-jun N-Terminal kinases are activated by ischaemia/reperfusion. Circ Res 79:162–173
Boucher FR, Hearse DJ, Opie LH (1994) Effects of trimetazidine on ischaemic contracture in isolated perfused rat heart. J Cardiovasc Pharmacol 24:45–49
Cokkinos DV (2001) Can metabolic manipulation reverse myocardial dysfunction? Eur Heart J 22:2138–2139
El Banani H, Bernard M, Baetz D, Cabanes E, Cozzone P, Lucien A, Feuvray D (2000) Changes in intracellular sodium and pH during ischaemia-reperfusion are attenuated by trimetazidine; comparison between low-and zero flow ischaemia. Cardiovasc Res 47:688–696
Hausenloy DJ, Yellon DM (2004) New directions for protecting the heart against ischemia-reperfusion injury: targeting the reperfusion injury salvage kinase (RISK)-pathway. Cardiovasc Res 61:448–460
Griffin JL, White LT, Lewandowski ED (2000) Substrate-dependent proton load and recovery of stunned hearts during pyruvate dehydrogenase stimulation. Am J Physiol Heart Circ Physiol 279 (1):H361–H367
Kantor PF, Lucien A, Kozak R, Lopaschuk GD (2000) The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long chain 3-ketoacyl coenzyme A thiolase. Circ Res 86:580–588
Knight RJ, Buxton DB (1996) Stimulation of c-Jun kinase and mitogen-activated protein kinase by ischaemia and reperfusion in the perfused rat heart. Biochem Biophys Res Commun 218:83–88
Kudej RK, White LT, Kudej AB, Vatner SF, Lewandowski ED (2002) Brief increase in carbohydrate oxidation after reperfusion reverses myocardial stunning in conscious pigs. Circulation 106:2836–2841
Lavanchy N, Martin J, Rossi A (1987) Antischaemic effects of trimetazidine: 31P–NMR spectroscopy in the isolated rat heart. Arch Int Pharmacodyn Ther 286:97–110
Liu B, Clanachan AS, Schulz R, Lopaschuk GD (1996) Cardiac ef.ciency is improved after ischaemia by altering both the source and fate of protons. Circ Res 79:940–948
Lopaschuk GD (1998) Treating ischaemic heart disease by pharmacologically improving cardiac energy metabolism. Am J Cardiol 82:14K–17K
Mackay K, Mochly-Rosen D (1999) An inhibitor of p38 mitogen-activated protein kinase protects neonatal cardiac myocytes from ischaemia. J Biol Chem 274 (10):6272–6279
Marais E, Genade S, Huisamen B, Strijdom JG, Moolman JA, Lochner A (2001) Activation of p38 MAPK induced by a multi-cycle ischaemic preconditioning protocol is associated with attenuated p38 MAPK activity during sustained ischaemia and reperfusion. J Mol Cell Cardiol 33:769–778
Marzilli M (2003) Cardioprotective effects of trimetazidine: a review. Curr Med Res Opin 19 (7):661–672
Maupoil V, Rochette L, Tabard A, Clauser P, Harpey C (1990) Direct measurement of free radical generation in isolated rat heart by electron paramagnetic resonance spectroscopy: effect of trimetazidine. Adv Exp Med Biol 264:377–382
McVeigh JJ, Lopaschuk GD (1990) Dichloroacetate stimulation of glucose oxidation improves recovery of ischemic rat hearts. Am J Physiol 259:H1079–H1085
Minners J, van der Boss EJ, Yellon DM, Schwalb H, Opie LH, Sack MN (2000) Dinitrophenol, cyclosporin A, and trimetazidine modulate preconditioning in the isolated rat heart: support for a mitochondrial role in cardioprotection. Cardiovasc Res 47:68–73
Opie LH (2003) Preconditioning and metabolic anti-ischaemic agents. Eur Heart J 24:1854–1856
Pantos C, Malliopoulou V, Mourouzis I, Karamanoli E, Paizis I, Steimberg N, Varonos D, Cokkinos DV (2002) Longterm thyroxine administration protects the heart in a similar pattern as ischaemic preconditioning. Thyroid 12:325–329
Pantos C, Malliopoulou V, Mourouzis I, Sfakianoudis K, Tzeis S, Doumba P, Xinaris Ch, Cokkinos AD, Carageorgiou H, Varonos D, Cokkinos DV (2003a) Propylthiouracil induced hypothyroidism is associated with increased tolerance of the isolated rat heart to ischaemia-reperfusion. J Endocrinol 178 (3):427–435
Pantos C, Malliopoulou V, Paizis I, Moraitis P, Mourouzis I, Tzeis S, Karamanoli E, Cokkinos DD, Carageorgiou H, Varonos D, Cokkinos DV (2003b) Thyroid hormone and cardioprotection; study of p38 MAPK and JNKs during ischaemia and at reperfusion in isolated rat heart. Mol Cell Biochem 242:173–180
Pantos C, Malliopoulou V, Mourouzis I, Moraitis P, Tzeis S, Thempeyioti A, Paizis I, Cokkinos AD, Carageorgiou H, Varonos D, Cokkinos DV (2003c) Involvement of p38 MAPK and JNK in the heat stress induced cardioprotection. Bas Res Cardiol 98:158–164
Pantos C, Malliopoulou V, Varonos D, Cokkinos DV (2004) Thyroid hormone and phenotypes of cardioprotection Basic Res Cardiol 99:101–120
Racey-Burns LA, Burns AH, Summer WR, Shepherd RE (1989) The effects of dichloroacetate on the isolated no flow arrested rat heart. Life Sci 44 (26):2015–2023
Renaud JF (1988) Internal pH, Na+ and Ca2+ regulation by trimetazidine during cardiac cell acidosis. Cardiovasc Drugs Ther 1:677–686
Stanley WC, Marzilli M (2003) Metabolic therapy in the treatment of ischaemic heart disease: the pharmacotherapy of trimetazidine. Fundamental Clinical Pharmacol 17:133–145
Taegtmeyer H, King LM, Jones BE (1998) Energy substrate metabolism, myocardial ischaemia, and targets for pharmacotherapy. Am J Cardiol 82:54K–60K
White LT, O’Donnell JM, Griffin J, Lewandowski ED (1999) Cytosolic redox state mediates postischaemic response to pyruvate dehydrogenase stimulation. Am J Physiol 277:H626–H634
Yellon DM, Downey JM (2003) Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev 83:1113–1151
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pantos, C., Bescond-Jacquet, A., Tzeis, S. et al. Trimetazidine protects isolated rat hearts against ischemia–reperfusion injury in an experimental timing – dependent manner. Basic Res Cardiol 100, 154–160 (2005). https://doi.org/10.1007/s00395-004-0505-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-004-0505-4