Abstract
Rationale
Atomoxetine, reboxetine and methylphenidate all act at the noradrenaline transporter (NAT) and atomoxetine and methylphenidate are licensed for the treatment of ADHD. The five-choice serial reaction time task (5CSRTT) provides a valid model to study attention and impulsivity in rodents. Studies using this task have largely failed to demonstrate improvements in attention with atomoxetine and methylphenidate and reboxetine has not been investigated previously.
Objectives
The present study used modifications to the standard rat 5CSRTT and demonstrated that blockade of NAT improves attention and reduces premature responding.
Methods
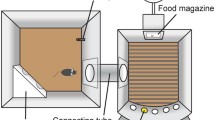
Rats were trained in a fixed inter-trial interval (ITI), 5CSRTT then tested at baseline and under conditions to acutely challenge attention and/or impulse control following vehicle or atomoxetine (0.3 mg/kg, i.p.).
Results
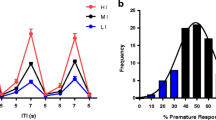
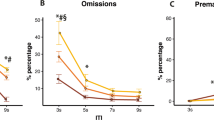
Atomoxetine (0.3 mg/kg, i.p.) significantly improved impulse control under all conditions (p < 0.05) but had no significant effects on accuracy. To increase the attentional demands of the task, rats were re-baselined in a non-paced, variable ITI task where presentations of the stimuli were unpredictable. In the VITI task, atomoxetine (0.0–0.3 mg/kg, i.p.) induced a dose-dependent improvement in accuracy (p < 0.05) and reduction in premature responses (p < 0.05). Reboxetine (0.0–1.0 mg/kg, i.p.) and methylphenidate (1–10 mg/kg, p.o.) did not significantly improve accuracy in the whole population but median split analysis revealed a significant improvement with both drugs, as well as atomoxetine, in the poor performing animals (p < 0.05).
Conclusions
These data suggest that blockade of noradrenaline re-uptake sites is an important target in terms of enhancing both attention and impulse control.



Similar content being viewed by others
References
Arnsten AF (2009) Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs 23(Suppl 1):33–41
Robbins TW, Arnsten AF (2009) The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci 32:267–287
Arnsten AF (2006) Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology 31:2376–2383
Wilens TE (2006) Mechanism of action of agents used in attention-deficit/hyperactivity disorder. J Clin Psychiatry 67(Suppl 8):32–38
Arnsten AF, Scahill L, Findling RL (2007) alpha2-Adrenergic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: emerging concepts from new data. J Child Adolesc Psychopharmacol 17:393–406
Kratochvil CJ, Vaughan BS, Harrington MJ, Burke WJ (2003) Atomoxetine: a selective noradrenaline reuptake inhibitor for the treatment of attention-deficit/hyperactivity disorder. Expert Opin Pharmacother 4:1165–1174
Kratochvil CJ, Wilens TE, Greenhill LL, Gao H, Baker KD, Feldman PD et al (2006) Effects of long-term atomoxetine treatment for young children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 45:919–927
Vaughan B, Fegert J, Kratochvil CJ (2009) Update on atomoxetine in the treatment of attention-deficit/hyperactivity disorder. Expert Opin Pharmacother 10:669–676
Arabgol F, Panaghi L, Hebrani P (2009) Reboxetine versus methylphenidate in treatment of children and adolescents with attention deficit-hyperactivity disorder. Eur Child Adolesc Psychiatry 18:53–59
Arnsten AF, Li BM (2005) Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry 57:1377–1384
Faraone SV, Mick E (2010) Molecular genetics of attention deficit hyperactivity disorder. Psychiatr Clin North Am 33:159–180
Arnsten AF, Dudley AG (2005) Methylphenidate improves prefrontal cortical cognitive function through alpha2-adrenoceptor and DA D1 receptor actions: relevance to therapeutic effects in attention deficit hyperactivity disorder. Behav Brain Func 1:2
Tripp G, Wickens JR (2009) Neurobiology of ADHD. Neuropharmacology 57:579–589
Curatolo P, Paloscia C, D'Agati E, Moavero R, Pasini A (2009) The neurobiology of attention deficit/hyperactivity disorder. Eur J Paediatr Neurol 13:299–304
Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B et al (2006) Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry 60:1111–1120
Devilbiss DM, Berridge CW (2006) Low-dose methylphenidate actions on tonic and phasic locus coeruleus discharge. J Pharmacol Exp Ther 319:1327–1335
Devilbiss DM, Berridge CW (2008) Cognition-enhancing doses of methylphenidate preferentially increase prefrontal cortex neuronal responsiveness. Biol Psychiatry 64:626–635
Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH et al (2002) Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacol 27:699–711
Swanson CJ, Perry KW, Koch-Krueger S, Katner J, Svensson KA, Bymaster FP (2006) Effect of the attention deficit/hyperactivity disorder drug atomoxetine on extracellular concentrations of norepinephrine and dopamine in several brain regions of the rat. Neuropharmacol 50:755–760
Carboni E, Silvagni A (2004) Dopamine reuptake by norepinephrine neurons: exception or rule? Crit Rev Neurobiol 16:121–128
Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ (2006a) Neurochemical modulation of response inhibition and probabilistic learning in humans. Science 311:861–863
Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X et al (2008) Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology 33:1028–1037
Bari A, Eagle DM, Mar AC, Robinson ES, Robbins TW (2009) Dissociable effects of NA, DA, and serotonin uptake blockade on stop task performance in rats. Psychopharmacology (Berl) 205:273–283
Blondeau C, Dellu-Hagedorn F (2007) Dimensional analysis of ADHD subtypes in rats. Biol Psychiatry 61:1340–1350
Navarra R, Graf R, Huang Y, Logue S, Comery T, Hughes Z et al (2008) Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog Neuropsychopharmacol Biol Psychiatry 32:34–41
Tsutsui-Kimura I, Ohmura Y, Izumi T, Yamaguchi T, Yoshida T, Yoshioka M (2009) The effects of serotonin and/or noradrenaline reuptake inhibitors on impulsive-like action assessed by the three-choice serial reaction time task: a simple and valid model of impulsive action using rats. Behav Pharmacol 20:474–483
Floresco SB, Jentsch JD (2011) Pharmacological enhancement of memory and executive functioning in laboratory animals. Neuropsychopharmacology 36:227–250
Robbins TW (2002) The 5-choice serial reaction time task: behavioral pharmacology and functional neurochemistry. Psychopharmacology 163:362–380
Milstein JA, Dalley JW, Robbins TW (2010) Methylphenidate-induced impulsivity: pharmacological antagonism by beta-adrenoreceptor blockade. J Psychopharmacol 24:309–321
Dalley JW, Cardinal RN, Robbins TW (2004) Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev 28:771–784
Chamberlain SR, Müller U, Robbins TW, Sahakian BJ (2006b) Neuropharmacological modulation of cognition. Curr Opin Neurol 19:607–612
Aston-Jones G, Cohen JD (2005) An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28:403–450
Liu YP, Lin YL, Chuang CH, Kao YC, Chang ST, Tung CS (2009) Alpha adrenergic modulation on effects of norepinephrine transporter inhibitor reboxetine in five-choice serial reaction time task. J Biomed Sci 16:72–76
Puumala T, Ruotsalainen S, Jäkälä P, Koivisto E, Riekkinen P Jr, Sirviö J (1996) Behavioral and pharmacological studies on the validation of a new animal model for attention deficit hyperactivity disorder. Neurobiol Learn Mem 66:198–211
Yamamoto BK, Novotney S (1998) Regulation of extracellular dopamine by the norepinephrine transporter. J Neurochem 71:274–280
Bizarro L, Patel S, Murtagh C, Stolerman IP (2004) Differential effects of psychomotor stimulants on attentional performance in rats: nicotine, amphetamine, caffeine and methylphenidate. Behav Pharmacol 15:195–206
Carli M, Robbins TW, Evenden JL, Everitt BJ (1983) Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res 9:361–380
Milstein JA, Lehmann O, Theobald DE, Dalley JW, Robbins TW (2007) Selective depletion of cortical noradrenaline by anti-dopamine beta-hydroxylase-saporin impairs attentional function and enhances the effects of guanfacine in the rat. Psychopharmacology 190:51–63
Bouret S, Sara SJ (2005) Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci 28:574–582
Robbins TW, Roberts AC (2007) Differential regulation of fronto-executive function by the monoamines and acetylcholine. Cereb Cortex 17(Suppl 1):151–160
Kehagia AA, Murray GK, Robbins TW (2010) Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol 20:199–204
Dalley JW, McGaughy J, O'Connell MT, Cardinal RN, Levita L, Robbins TW (2001) Distinct changes in cortical acetylcholine and noradrenaline efflux during contingent and noncontingent performance of a visual attentional task. J Neurosci 21:4908–4914
Tait DS, Brown VJ, Farovik A, Theobald DE, Dalley JW, Robbins TW (2007) Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. Eur J Neurosci 25:3719–3724
Oades RD, Sadile AG, Sagvolden T, Viggiano D, Zuddas A, Devoto P, Aase H, Johansen EB, Ruocco LA, Russell VA (2005) The control of responsiveness in ADHD by catecholamines: evidence for dopaminergic, noradrenergic and interactive roles. Dev Sci 8:122–131
Koffarnus MN, Katz JL (2011) Response requirement and increases in accuracy produced by stimulant drugs in a 5-choice serial reaction-time task in rats. Psychopharmacology 213:723–733
Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW (2003) Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res 146:105–119
Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW (2000) Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci 20:1208–1215
Gamo NJ, Wang M, Arnsten AF (2010) Methylphenidate and atomoxetine enhance prefrontal function through α2-adrenergic and dopamine D1 receptors. J Am Acad Child Adolesc Psychiatry 49:1011–1023
Cole BJ, Robbins TW (1989) Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi on performance of a 5-choice serial reaction time task in rats: implications for theories of selective attention and arousal. Behav Brain Res 33:165–179
Delfs JM, Zhu Y, Druhan JP, Aston-Jones GS (1998) Origin of noradrenergic afferents to the shell subregion of the nucleus accumbens: anterograde and retrograde tract-tracing studies in the rat. Brain Res 806:127–140
Acknowledgements
The author would like to thank Prof. David Nutt and Prof. Trevor Robbins for their valued discussion of this work, Sian Cundy for technical assistance and Nicholas Donnelly (Wellcome summer studentship) and Christian Wood (Nuffield summer studentship) for their help with running some of the experiments included in this manuscript. Funding for this research was provided by a Medical Research Council New Investigator grant (Ref: G0700980) awarded to E.S.J.R.
Conflict of interest
The author of this manuscript has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robinson, E.S.J. Blockade of noradrenaline re-uptake sites improves accuracy and impulse control in rats performing a five-choice serial reaction time tasks. Psychopharmacology 219, 303–312 (2012). https://doi.org/10.1007/s00213-011-2420-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2420-3