Abstract
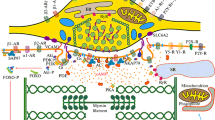
Electrical intercellular communication in the heart allows the propagation of an action potential from cell to cell. This is realized by low-ohmic cell-to-cell channels, the gap junction channels, which are dodecameric proteins consisting of two hexameric hemichannels. Each of the neighbouring cell provides one hemichannel, which consists of six connexins. In the heart, the connexin isoforms Cx43, Cx40 and Cx45 are present with Cx43 being the most abundant isoform. This intercellular communication is regulated acutely by control of the gap junction conductance and chronically by control of the connexin expression. The short half-life time of Cx43 indicates the permanent adaptation of cell communication to the actual requirements. β-Adrenoceptor stimulation enhances Cx40- but reduces Cx45-conductance, while Cx43 channels in most species do not seem to be acutely affected by β-adrenoceptor signalling. In contrast, chronic exposure to β-adrenergic stimulants activates protein kinase A and the mitogenic-activated protein kinase cascade (including protein 38 (p38), mitogenic-activated protein kinase kinase 1, extracellular signal-regulated kinase (ERK)1/2 and c-JUN NH2 terminal kinase (JNK)), the calcineurin pathway, translocation of activator protein 1 (AP1), CRE-binding protein and nuclear factor of activated T cells, finally leading to enhanced Cx43-mRNA and Cx43-protein expression together with Cx43 phosphorylation, but does not affect Cx40. α-Adrenoceptors also play a role in controlling cardiac intercellular communication: α-adrenergic stimulation acutely uncouples the cells, while a chronic stimulation enhances Cx43 expression via protein kinase C, p38, ERK1/2, JNK, c-fos and AP1, but does not alter Cx40 expression. While general cardiac protein synthesis, e.g. of β-actin, is controlled via α1A-adrenoceptors, Cx43 expression is regulated via α1D-adrenoceptors. However, α-adrenoceptor density in the heart varies among species, with high abundance in rat heart and low in human heart. Acute α-adrenergic stimulation, e.g. during ischemia, can lead to uncoupling and facilitates re-entrant arrhythmia. Chronic adrenergic upregulation of Cx43 expression seems to be involved in cardiac hypertrophy. In maladaptive hypertrophy, the enhanced Cx43 is increasingly incorporated in the lateral membrane of the cells rather at the cell poles, which may mean a gap junction disarray. This could—together with a mismatch in cell size and coupling—contribute to arrhythmogenesis. Thus, cardiac adrenoceptors are directly involved in the control of intercellular electrical communication and thus probably are a critical factor in the maintenance of regular cell-to-cell conduction and of the cardiac electrical networking. They probably are involved in the formation of an arrhythmogenic substrate in certain heart diseases.



Similar content being viewed by others
References
Ai X, Pogwizd SM (2005) Connexin 43 downregulation and dephosphorylation in nonischemic heart failure is associated with enhanced colocalized protein phosphatase type 2A. Circ Res 96:54–63
Aggeli IKS, Gaitanaki C, Beis I (2006) Involvement of JNKs and p38-MAPK/MSK1 pathways in H2O2-induced upregulation of heme oxygenase-1 mRNA in H9c2 cells. Cell Signal 18:1801–1812
Alcoléa S, Théveniau-Ruissy M, Jarry-Guichard T, Marics I, Tzouanacou E, Chauvin JP, Briand JP, Moorman AF, Lamers WH, Gros DB (1999) Downregulation of connexin 45 gene products during mouse heart development. Circ Res 84:1365–1379
Bailey J, Phillips RJ, Pollard AJ, Gilmore K, Robson SC, Europe-Finner GN (2002) Characterization and functional analysis of cAMP response element modulator protein and activating transcription factor 2 (ATF2) isoforms in the human myometrium during pregnancy and labor: identification of a novel ATF2 species with potent transactivation properties. J Clin Endocrinol Metab 87:1717–1728
Barker RJ, Price RL, Gourdie RG (2002) Increased association of ZO-1 with connexin43 during remodeling of cardiac gap junctions. Circ Res 90:317–324
Barki-Harrington L, Perrino C, Rockman HA (2004) Network integration of the adrenergic system in cardiac hypertrophy. Cardiovasc Res 63:391–402
Barthel F, Loeffler JP (1995) β2-Adrenoreceptors stimulate c-fos transcription through multiple cyclic AMP- and Ca2+ -responsive elements in cerebellar granular neurons. J Neurochem 64:41–51
Beardslee MA, Laing JG, Beyer EC, Saffitz JE (1998) Rapid turnover of connexin 43 in the adult rat heart. Circ Res 83:629–653
Bergoffen J, Scherer SS, Wang S, Oronzi-Scott M, Bone JL, Paul DL, Chen K, Lensch WM, Chance PF, Fischbeck KH (1993) Connexin mutations in X-linked Charcot–Marie–Tooth disease. Science 262:2039–2042
Bolon ML, Peng T, Kidder GM, Tyml K (2008) Lipopolysaccharide plus hypoxia and reoxygenation synergistically reduce electrical coupling between microvascular endothelial cells by dephosphorylating connexin40. J Cell Physiol 217:350–359
Boogerd KJ, Wong LY, Christoffels VM, Klarenbeek M, Ruijter JM, Moorman AF, Barnett P (2008) Msx1 and Msx2 are functional interacting partners of T-box factors in the regulation of Connexin43. Cardiovasc Res 78:485–493
Boutillier AL, Barthel F, Robertsll JL, Loeffler JP (1992) β-Adrenergic stimulation of cFOS via protein kinase A is mediated by cAMP regulatory element binding protein (CREB)-dependent and tissue-specific CREB-independent mechanisms in corticotrope cells. J Biol Chem 267:23520–23526
Bouvier D, Spagnol G, Chenavas S, Kieken F, Vitrac H, Brownell S, Kellezi A, Forge V, Sorgen PL (2009) Characterization of the structure and intermolecular interactions between the connexin40 and connexin43 carboxyl-terminal and cytoplasmic loop domains. J Biol Chem 284:34257–34271
Boyett MR, Inada S, Yoo S, Li J, Liu J, Tellez J, Greener ID, Honjo H, Billeter R, Lei M, Zhang H, Efimov IR, Dobrzynski H (2006) Connexins in the sinoatrial and atrioventricular nodes. In: Dhein S (ed) Cardiovascular gap junctions. Advances in cardiology, vol 42. Karger, Basel, pp 175–197
Bristow MR, Minobe W, Rasmussen R, Hershberger RE, Hoffman BB (1988) Alpha-1 adrenergic receptors in the nonfailing and failing human heart. J Pharmacol Exp Ther 247:1039–1045
Brodde OE, Michel MC (1999) Adrenergic and muscarinic receptors in the human heart. Pharmacol Rev 51:651–690
Burt JM, Fletcher AM, Steele TD, Wu Y, Cottrell GT, Kurjiaka DT (2001) Alteration of Cx43:Cx40 expression ratio in A7r5 cells. Am J Physiol Cell Physiol 280:C500–C508
Burt JM, Spray DC (1988) Inotropic agents modulate gap junctional conductance between cardiac myocytes. Am J Physiol 254:H1206–H1210
Camelliti P, Green CR, LeGrice I, Kohl P (2004) Fibroblast network in rabbit sinoatrial node: structural and functional identification of homogeneous and heterogeneous cell coupling. Circ Res 94:828–835
Campos de Carvalho AC, Tanowitz HB, Wittner M, Dermietzel R, Roy C, Hertzberg EL, Spray DC (1992) Gap junction distribution is altered between cardiac myocytes infected with Trypanosoma cruzi. Circ Res 70:733–742
Carroll WA, Sippy KB, Esbenshade TA, Buckner SA, Hancock AA, Meyer MD (2001) Two novel and potent 3-[(o-methoxyphenyl)piperazinylethyl]-5-phenylthien. Bioorg Med Chem Lett 11:1119–1121
Caterall WA (1988) Structure and function of voltage sensitive channels. Science 242:50–61
Chang DJ, Chang TK, Yamanishi SS, Salazar FH, Kosaka AH, Khare R, Bhakta S, Jasper JR, Shieh IS, Lesnick JD, Ford AP, Daniels DV, Eglen RM, Clarke DE, Bach C, Chan HW (1998) Molecular cloning, genomic characterization and expression of novel human alpha1A-adrenoceptor isoforms. FEBS Lett 422:279–283
Chen L, Goings GE, Upshaw-Earley J, Page E (1989) Cardiac gap junctions and gap junction-associated vesicles: ultrastructural comparison of in situ negative staining with conventional positive staining. Circ Res 64:501–514
Christ GJ, Spray DC, El-Sabban M, Moore LK, Brink PR (1996) Gap junctions in vascular tissues. Evaluating the role of intercellular communication in the modulation of vasomotor tone. Circ Res 79:631–646
Coutinho P, Qiu C, Frank S, Tamber K, Becker D (2003) Dynamic changes in connexin expression correlate with key events in the wound healing process. Cell Biol Int 27:525–541
Daaka Y, Luttrell LM, Lefkowitz RJ (1997) Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature 390:88–91
Darrow BJ, Fast VG, Kléber AG, Beyer EC, Saffitz JE (1996) Functional and structural assessment of intercellular communication. Increased conduction velocity and enhanced connexin expression in dibutyryl cAMP-treated cultured cardiac myocytes. Circ Res 79:174–183
Darrow BJ, Laing JG, Lampe PD, Saffitz JE, Beyer EC (1995) Expression of multiple connexins in cultured neonatal rat ventricular cardiomyocytes. Circ Res 76:381–387
Dasgupta C, Martinez AM, Zuppan CW, Shah MM, Bailey LL, Fletcher WH (2001) Identification of connexin43 (alpha1) gap junction gene mutations in patients with hypoplastic left heart syndrome by denaturing gradient gel electrophoresis (DGGE). Mutat Res 479:173–186
Davies TC, Barr KJ, Jones DH, Zhu D, Kidder GM (1996) Multiple members of the connexin gene family participate in preimplantation development of the mouse. Dev Gen 18:234–243
Davis LM, Rodefeld ME, Green K, Beyer EC, Saffitz JE (1995) Gap junction protein phenotypes of the human heart and conduction system. J Cardiovasc Electrophysiol 6:813–822
De Boer TP, van Rijen HV, Van der Heyden MA, Kok B, Opthof T, Vos MA, Jongsma HJ, de Bakker JM, van Veen TA (2007) Beta-, not alpha-adrenergic stimulation enhances conduction velocity in cultures of neonatal cardiomyocytes. Circ J 71:973–981
De Groot JR, Wilms-Schopman FJ, Opthof T, Remme CA, Coronel R (2001) Late ventricular arrhythmias during acute regional ischemia in the isolated blood perfused pig heart. Role of electrical cellular coupling. Cardiovasc Res 50:362–372
Dekker LRC, Fiolet JWT, VanBavel E, Coronel R, Opthof T, Spaan JAE, Janse MJ (1996) Intracellular Ca2+, intercellular electrical coupling and mechanical activity in ischemic rabbit papillary muscle. Effects of preconditioning and metabolic blockade. Circ Res 79:237–246
De Leon JR, Buttrick PM, Fishman GI (1994) Functional analysis of the connexin43 gene promoter in vivo and in vitro. J Mol Cell Cardiol 26:379–389
De Mello WC (1984) Effect of intracellular injection of cAMP on the electrical coupling of mammalian cardiac cells. Biochem Biophys Res Comm 119:1001–1007
De Mello WC (1991) Cyclic AMP and junctional communication viewed through a multi-biophysical approach. In: Peracchia C (ed) Biophysics of gap junction channels. CRC, Boca Raton, pp 229–239
De Mello WC (1996) Impaired regulation of cell communication by beta-adrenergic receptor activation in the failing heart. Hypertension 27:265–268
De Mello WC (1997) Influence of alpha-adrenergic receptor activation on junctional conductance in heart cells: interaction with beta-adrenergic agonists. J Cardiovasc Pharmacol 29:273–277
Dhein S (1998) Gap junction channels in the cardiovascular system: pharmacological and physiological modulation. Trends Pharmacol Sci 19:229–241
Dhein S (2006) Cardiac ischemia and uncoupling: gap junctions in ischemia and infarction. Adv Cardiol 42:198–212
Dhein S, Hammerrath SB (2001) Aspects of the intercellular communication in aged hearts. Effects of the gap junction uncoupler palmitoleic acid. Naunyn Schmiedebergs Arch Pharmacol 364:397–408
Dhein S, Krüsemann K, Schaefer T (1999) Effects of the gap junction uncoupler palmitoleic acid on the activation and repolarization wavefronts in isolated rabbit hearts. Br J Pharmacol 128:1375–1384
Doble BW, Kardami E (1995) Basic fibroblast growth factor stimulates connexin-43 expression and intercellular communication of cardiac fibroblasts. Mol Cell Biochem 143:81–87
Dodge SM, Beardslee MA, Darrow BJ, Green KG, Beyer EC, Saffitz JE (1998) Effects of angiotensin II on expression of the gap junction channel protein connexin43 in neonatal rat ventricular myocytes. J Am Coll Cardiol 32:800–807
Doggrell SA, Petcu EB, Barnett CW (1998) Affinity constants and beta-adrenoceptor reserves for isoprenaline on cardiac tissue from normotensive and hypertensive rats. J Pharm Pharmacol 50:215–223
Dupays L, Mazurais D, Rücker-Martin C, Calmels T, Bernot D, Cronier L, Malassiné A, Gros D, Théveniau-Ruissy M (2003) Genomic organization and alternative transcripts of the human Connexin40 gene. Gene 305:79–90
Duthe F, Plaisance I, Sarrouilhe D, Hervé JC (2001) Endogenous protein phosphatase 1 runs down gap junctional communication of rat ventricular myocytes. Am J Physiol 281:C1648–C1656
Echetebu CO, Ali M, Izban MG, MacKay L, Garfield RE (1999) Localization of regulatory protein binding sites in the proximal region of human myometrial connexin 43 gene. Mol Hum Reprod 5:757–766
Engelmann TW (1875) Über die Leitung der Erregung im Herzmuskel. Arch Gesamte Physiol 11:465–480
Evans WH, De Vuyst E, Leybaert L (2006) The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem J 397(1):1–14
Falk MM (2000) Biosynthesis and structural composition of gap junction intercellular membrane channels. Eur J Cell Biol 79:564–574
Fan GC, Yuan Q, Song G, Wang Y, Chen G, Qian J, Zhou X, Lee YJ, Ashraf M, Kranias EG (2006) Small heat-shock protein Hsp20 attenuates beta-agonist-mediated cardiac remodeling through apoptosis signal-regulating kinase 1. Circ Res 99:1233–1242
Ford AP, Daniels DV, Chang DJ, Gever JR, Jasper JR, Lesnick JD, Clarke DE (1997) Pharmacological pleiotropism of the human recombinant α1A-adrenoceptor: implications for α1-adrenoceptor classification. Br J Pharmacol 121:1127–1135
Frey N, Olson EN (2003) Cardiac hypertrophy: the good, the bad and the ugly. Annu Rev Physiol 65:45–79
Gaietta G, Deerinck TJ, Adams SR, Bouwer J, Tour O, Laird DW, Sosinsky GE, Tsien RY, Ellisman MH (2002) Multicolor and electron microscopic imaging of connexin trafficking. Science 296:503–507
Geimonen E, Jiang W, Ali M, Fishman GI, Garfield RE, Andersen J (1996) Activation of protein kinase C in human uterine smooth muscle induces connexin-43 gene transcription through an AP-1 site in the promoter sequence. J Biol Chem 271:23667–23674
Giepmans BN (2004) Gap junctions and connexin interacting proteins. Cardiovasc Res 62:233–245
Glover D, Little JB, Lavin MF, Gueven N (2003) Low dose ionizing radiation-induced activation of connexin 43 expression. Int J Radiat Biol 79:955–964
Goldspink PH, Russell B (1996) Physiological role of phosphorylation of the cyclic AMP response element binding protein in rat cardiac nuclei. Cell Tissue Res 285:379–385
Hervé JC, Dhein S (2006) Pharmacology of cardiovascular gap junctions. Adv Cardiol 42:107–131
Heubach JF, Ravens U, Kaumann AJ (2004) Epinephrine activates both Gs and Gi pathways, but norepinephrine activates only the Gs pathway through human beta2-adrenoceptors overexpressed in mouse heart. Mol Pharmacol 65:1313–1322
Hoffmann C, Leitz MR, Oberdorf-Maass S, Lohse MJ, Klotz KN (2004) Comparative pharmacology of human beta-adrenergic receptor subtypes—characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch Pharmacol 369:151–159
Honjo H, Boyett MR, Coppen SR, Takagishi Y, Opthof T, Severs NJ, Kodama I (2002) Heterogeneous expression of connexins in rabbit sinoatrial node cells: correlation between connexin isotype and cell size. Cardiovasc Res 53:89–96
Jacob A, Beyer EC (2001) Mouse connexin 45: genomic cloning and exon usage. DNA Cell Biol 20:11–19
Jongsma HJ, Wilders R (2000) Gap junctions in cardiovascular disease. Circ Res 86:1193–1197
Jordan K, Chodock R, Hand AR, Laird DW (2001) The origin of annular junctions: a mechanism of gap junction internalization. J Cell Sci 114:763–773
Kaplan SR, Gard JJ, Protonotarios N, Tsatsopoulou A, Spiliopoulou C, Anastasakis A, Squarcioni CP, McKenna WJ, Thiene G, Basso C, Brousse N, Fontaine G, Saffitz JE (2004) Remodeling of myocyte gap junctions in arrhythmogenic right ventricular cardiomyopathy due to a deletion in plakoglobin (Naxos disease). Heart Rhythm 1:3–11
Kemp BE, Pearson RB (1990) Protein kinase recognition sequence motifs. Trends Biochem Sci 15:342–346
Kennely PJ, Krebs PG (1991) Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem 266:15555–15558
Kostin S, Dammer S, Hein S, Klövekorn WP, Bauer EP, Schaper J (2004) Connexin 43 expression and distribution in compensated and decompensated cardiac hypertrophy in patients with aortic stenosis. Cardiovasc Res 62:426–436
Kostin S, Rieger M, Dammer S, Hein S, Richter M, Klövekorn WP, Bauer EP, Schaper J (2003) Gap junction remodeling and altered connexin43 expression in the failing human heart. Mol Cell Biochem 242:135–144
Krishnamurthy P, Subramanian V, Singh M, Singh K (2007) Beta1 integrins modulate beta-adrenergic receptor-stimulated cardiac myocyte apoptosis and myocardial remodeling. Hypertension 49:865–872
Kwak BR, Hermans MM, De Jonge HR, Lohmann SM, Jongsma HJ, Chanson M (1995) Differential regulation of distinct types of gap junction channels by similar phosphorylating conditions. Mol Biol Cell 6:1707–1719
Kwak BR, Jongsma HJ (1996) Regulation of cardiac gap junction channel permeability and conductance by several phosphorylating conditions. Mol Cell Biochem 157:93–99
Kwak BR, Mulhaupt F, Veillard N, Gros DB, Mach F (2002) Altered pattern of vascular connexin expression in atherosclerotic plaques. Arterioscler Thromb Vasc Biol 22:225–230
Laing JG, Beyer EC (1995) The gap junction protein connexin43 is degraded via the ubiquitin proteasome pathway. J Biol Chem 270:26399–26403
Laird DW, Puranam KL, Revel JP (1991) Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochem J 273:67–72
Lampe PD (1994) Analyzing phorbol ester effects on gap junctional communication: a dramatic inhibition of assembly. J Cell Biol 127:1895–1905
Lampe PD, Lau AF (2003) The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol 36:1171–1186
Lauf U, Giepmans BN, Lopez P, Braconnot S, Chen SC, Falk MM (2002) Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci USA 99:10446–10451
Lee PJ, Pogwizd SM (2006) Micropatterns of propagation. Adv Cardiol 42:86–106
Lin R, Warn-Cramer BJ, Kurata WE, Lau AF (2001) v-Src phosphorylation of connexin 43 on Tyr247 and Tyr265 disrupts gap junctional communication. J Cell Biol 154:815–827
Loewenstein WR (1981) Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev 61:829–913
Loewenstein WR, Kanno Y (1966) Intercellular communication and the control of tissue growth: lack of communication between cancer cells. Nature 209:248–1249
Louault C, Benamer N, Faivre JF, Potreau D, Bescond J (2008) Implication of connexins 40 and 43 in functional coupling between mouse cardiac fibroblasts in primary culture. Biochim Biophys Acta 1778:2097–2104
Markou T, Hadzopoulou-Cladaras M, Lazou A (2004) Phenylephrine induces activation of CREB in adult rat cardiac myocytes through MSK1 and PKA signaling pathways. J Mol Cell Cardiol 37:1001–1011
Maurer P, Weingart R (1987) Cell pairs isolated from guinea pig and rat hearts: effects of [Ca++]i on nexal membrane resistance. Eur J Physiol 409:394–402
Meyer RA, Laird DW, Revel JP, Johnson RG (1992) Inhibition of gap junction and adherens junction assembly by connexin and A-CAM antibodies. J Cell Biol 119:179–189
Moreno AP, Fishman GI, Spray DC (1992) Phosphorylation shifts unitary conductance and modifies voltage dependent kinetics of human connexin43 gap junction channels. Biophys J 62:51–53
Morley GE, Vaidya D, Samie FH, Lo C, Delmar M, Jalife J (1999) Characterization of conduction in the ventricles of normal and heterozygous Cx43 knockout mice using optical mapping. J Cardiovasc Electrophysiol 10:1361–1375
Müller FU, Boknik P, Knapp J, Linck B, Lüss H, Neumann J, Schmitz W (2001) Activation and inactivation of cAMP-response element-mediated gene transcription in cardiac myocytes. Cardiovasc Res 52:95–102
Müller A, Gottwald M, Tudyka T, Linke W, Klaus W, Dhein S (1997) Increase in gap junction conductance by an antiarrhythmic peptide. Eur J Pharmacol 327:65–72
Musil LS, Cunningham BA, Edelman GM, Goodenough DA (1990) Differential phosphorylation of the gap junction protein connexin43 in junctional communication-competent and -deficient cell lines. J Cell Biol 111:2077–2088
Musil LS, Goodenough DA (1991) Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol 115:1357–1374
Nielsen PA, Kumar NM (2003) Differences in expression patterns between mouse connexin-30.2 (Cx30.2) and its putative human orthologue, connexin-31.9. FEBS Lett 540:151–156
Noble BS, Reeve J (2000) Osteocyte function, osteocyte death and bone fracture resistance. Mol Cell Endocrinol 159:7–13
Paulson AF, Lampe PD, Meyer RA, TenBroek E, Atkinson MM, Walseth TF, Johnson RG (2000) Cyclic AMP and LDL trigger a rapid enhancement in gap junction assembly through a stimulation of connexin trafficking. J Cell Sci 113:3037–3049
Peters NS, Severs NJ, Rothery SM, Lincoln C, Yacoub MH, Green CR (1994) Spatiotemporal relation between gap junctions and fascia adherens junctions during postnatal development of human ventricular myocardium. Circulation 90:713–725
Pfaff M, Liu S, Erle DJ, Ginsberg MH (1998) Integrin beta cytoplasmic domains differentially bind to cytoskeletal proteins. J Biol Chem 273:6104–6109
Pfeifer I, Anderson C, Werner R, Oltra E (2004) Redefining the structure of the mouse connexin43 gene: selective promoter usage and alternative splicing mechanisms yield transcripts with different translational efficiencies. Nucleic Acids Res 32:4550–4562
Pönicke K, Schlüter K-D, Heinroth-Hoffmann I, Seyfarth T, Goldberg M, Osten B, Piper HM, Brodde OE (2001) Noradrenaline-induced increase in protein synthesis in adult rat cardiomyocytes: involvement of only alpha1A-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol 364:444–453
Polontchouk L, Ebelt B, Jackels M, Dhein S (2002) Chronic effects of endothelin 1 and angiotensin II on gap junctions and intercellular communication in cardiac cells. FASEB J 16:87–89
Polontchouk L, Haefliger J-A, Ebelt B, Schaefer T, Stuhlmann D, Mehlhorn U, Kuhn-Reignier F, DeVivie ER, Dhein S (2001) Effects of chronic atrial fibrillation on gap junction distribution in human and rat atria. J Am Coll Cardiol 38:883–891
Puranam KL, Laird DW, Revel JP (1993) Trapping an intermediate form of connexin43 in the Golgi. Exp Cell Res 206:85–92
Qin H, Shao Q, Igdoura SA, Alaoui-Jamali MA, Laird DW (2003) Lysosomal and proteasomal degradation play distinct roles in the life cycle of Cx43 in gap junctional intercellular communication-deficient and -competent breast tumor cells. J Biol Chem 278:30005–30014
Ramirez MT, Sah VP, Zhao XL, Hunter JJ, Chien KR, Brown JH (1997) The MEKK-JNK pathway is stimulated by alpha1-adrenergic receptor and ras activation and is associated with in vitro and in vivo cardiac hypertrophy. J Biol Chem 272:14057–14061
Rohde S, Sabri A, Kamasamudran R, Steinberg SF (2000) The alpha(1)-adrenoceptor subtype- and protein kinase C isoform-dependence of norepinephrine’s actions in cardiomyocytes. J Mol Cell Cardiol 32:1193–1209
Rohr S (2004) Role of gap junctions in the propagation of the cardiac action potential. Cardiovasc Res 62:309–322
Rohr S, Kucera JP, Fast VG, Kleber AG (1997) Paradoxical improvement of impulse conduction in cardiac tissue by partial cellular uncoupling. Science 275:841–844
Rojas Gomez DM, Schulte JS, Mohr FW, Dhein S (2008) Alpha-1-adrenoceptor subtype selective regulation of connexin 43 expression in rat cardiomyocytes. Naunyn Schmiedebergs Arch Parmacol 377:77–85
Ross RS (2004) Molecular and mechanical synergy: cross-talk between integrins and growth factor receptors. Cardiovasc Res 63:381–390
Rutkowski R, Kosztyła-Hojna B, Kańczuga-Koda L, Sulkowska M, Sulkowski S, Rutkowski K (2008) Structure and physiological function of connexin proteins. Postepy Hig Med Dosw 62:632–641, Polish
Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC (2003) Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev 83:1359–1400
Salameh A, Dhein S (2005) Pharmacology of gap junctions. New pharmacological targets for treatment of arrhythmia, seizure and cancer? Biochim Biophys Acta 1719(1–2):36–58
Salameh A, Frenzel C, Boldt A, Rassler B, Glawe I, Schulte J, Muhlberg K, Zimmer HG, Pfeiffer D, Dhein S (2006) Subchronic alpha- and beta-adrenergic regulation of cardiac gap junction protein expression. FASEB J 20:365–367
Salameh A, Krautblatter S, Baessler S, Karl S, Rojas Gomez D, Dhein S, Pfeiffer D (2008) Signal transduction and transcriptional control of cardiac connexin43 up-regulation after alpha1-adrenoceptor stimulation. J Pharmacol Exp Ther 326:315–322
Salameh A, Krautblatter S, Karl S, Blanke K, Rojas Gomez D, Dhein S, Pfeiffer D, Janousek J (2009) The signal transduction cascade regulating the expression of the gap junction protein connexin43 by β-adrenoceptors. Brit J Pharmacol 158:198–208
Salameh A, Wustmann A, Karl S, Blanke K, Apel D, Rojas-Gomez D, Franke H, Mohr FW, Janousek J, Dhein S (2010) Cyclic mechanical stretch induces cardiomyocyte orientation and polarization of the gap junction protein Connexin43. Circ Res 106:1592–1602
Samoilova M, Li J, Pelletier MR, Wentlandt K, Adamchik Y, Naus CC, Carlen PL (2003) Epileptiform activity in hippocampal slice cultures exposed chronically to bicuculline: increased gap junctional function and expression. J Neurochem 86:687–699
Schlüter KD, Piper HM (1992) Trophic effects of catecholamines and parathyroid hormone on adult ventricular cardiomyocytes. Am J Physiol Heart Circ Physiol 263:H1739–H1746
Schlüter KD, Simm A, Schäfer M, Taimor G, Piper HM (1999) Early response kinase and PI 3-kinase activation in adult cardiomyocytes and their role in hypertrophy. Am J Physiol Heart Circ Physiol 276:H1655–H1663
Schreckenberg R, Taimor G, Piper HM, Schlüter KD (2004) Inhibition of Ca2+-dependent PKC isoforms unmasks ERK-dependent hypertrophic growth evoked by phenylephrine in adult ventricular cardiomyocytes. Cardiovasc Res 63:553–560
Schulte JS, Salameh A, Mohr FW, Dhein S (2007) Akute versus chronische Regulation von kardialen Gap Junctions durch Phenylehrin und Isoprenalin. Clin Res Cardiol 96(suppl 1):V1115
Segretain D, Falk MM (2004) Regulation of connexin biosynthesis, assembly, gap junction formation, and removal. Biochim Biophys Acta 1662:3–21
Seidel T, Salameh A, Dhein S (2010) A simulation study of cellular hypertrophy and connexin lateralization in cardiac tissue. Biophys J 99:2821–2830
Selvetella G, Hirsch E, Notte A, Tarone G, Lembo G (2004) Adaptive and maladaptive hypertrophic pathways: points of convergence and divergence. Cardiovasc Res 63:373–380
Shaw RM, Rudy Y (1997) Ionic mechanisms of propagation in cardiac tissue. Roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res 81:727–741
Simpson I, Rose B, Loewenstein WR (1977) Size limit of molecules permeating the junctional membrane channels. Science 195:294–296
Söhl G, Willecke K (2003) An update on connexin genes and their nomenclature in mouse and man. Cell Commun Adhes 10:73–80
Spach MS, Miller WT, Dolber PC, Kootsey JM, Sommer JR, Mosher CE (1982) The functional role of structural complexities in the propagation of depolarization in the atrium of the dog. Cardiac conduction disturbances due to discontinuities of effective axial resistivity. Circ Res 50:175–191
Taimor G, Schlüter KD, Best P, Helmig S, Piper HM (2004) Transcription activator protein 1 mediates alpha- but not beta-adrenergic hypertrophic growth responses in adult cardiomyocytes. Am J Physiol Heart Circ Physiol 286:H2369–H2375
Taniguchi T, Inagaki R, Murata S, Akiba I, Muramatsu I (1999) Microphysiometric analysis of human alpha1a-adrenoceptor expressed in Chinese hamster ovary cells. Br J Pharmacol 127:962–968
TenBroek EM, Lampe PD, Solan JL, Reynhout JK, Johnson RG (2001) Ser364 of connexin43 and the upregulation of gap junction assembly by cAMP. J Cell Biol 155:1307–1318
Teunissen BE, Bierhuizen MF (2004) Transcriptional control of myocardial connexins. Cardiovasc Res 62:246–255
Teunissen BE, Jansen AT, van Amersfoorth SC, O'Brien TX, Jongsma HJ, Bierhuizen MF (2003) Analysis of the rat connexin 43 proximal promoter in neonatal cardiomyocytes. Gene 322:123–136
Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M (1998) Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem 273:12725–12731
Trosko JE (2003) The role of stem cells and gap junction intercellular communication in carcinogenesis. J Biochem Mol Biol 36:43–48
Unwin PNT, Zampighi G (1980) Structure of the junction between communicating cells. Nature 283:545–549
Valiunas V, Gemel J, Brink PR, Beyer EC (2001) Gap junction channels formed by coexpressed connexin40 and connexin43. Am J Physiol Heart Circ Physiol 281:H1675–H1689
Van Rijen HV, van Veen TA, Hermans MM, Jongsma HJ (2000) Human connexin40 gap junction channels are modulated by cAMP. Cardiovasc Res 45:941–951
Van Veen TAB, van Rijen HV, Jongsma HJ (2000) Electrical conductance of mouse connexin45 gap junction channels is modulated by phosphorylation. Cardiovasc Res 46:496–510
Van Veen TAB, van Rijen HV, Jongsma HJ (2005a) Physiology of cardiovascular gap junctions. Adv Cardiol 42:18–40
van Veen TA, van Rijen HV, van Kempen MJ, Miquerol L, Opthof T, Gros D, Vos MA, Jongsma HJ, de Bakker JM (2005b) Discontinuous conduction in mouse bundle branches is caused by bundle-branch architecture. Circulation 112:2235–2244
Warner A, Clements DK, Parikh S, Evans WH, DeHaan RL (1995) Specific motifs in the external loops of connexin proteins can determine gap junction formation between chick heart myocytes. J Physiol Lond 488:721–728
Weber PA, Chang HC, Spaeth KE, Nitsche JM, Nicholson BJ (2004) The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophys J 87:958–973
Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Güldenagel M, Deutsch U, Söhl G (2002) Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem 383:725–737
Willecke K, Jungbluth S, Dahl E, Hennemann H, Heynkes R, Grzeschik KH (1990) Six genes of the human connexin gene family coding for gap junctional proteins are assigned to four different human chromosomes. Eur J Cell Biol 53:275–280
Wu JC, Tsai RY, Chung TH (2003) Role of catenins in the development of gap junctions in rat cardiomyocytes. J Cell Biochem 88:823–835
Xiao L, Pimental DR, Amin JK, Singh K, Sawyer DB, Colucci WS (2001) MEK1/2-ERK1/2 mediates alpha1-adrenergic receptor stimulated hypertrophy in adult rat ventricular myocytes. J Mol Cell Cardiol 33:779–787
Yoshio R, Taniguchi T, Itoh H, Muramatsu I (2001) Affinity of serotonin receptor antagonists and agonists to recombinant and native alpha1-adrenoceptor subtypes. Jpn J Pharmacol 86:189–195
Zhang GX, Kimura S, Nishiyama A, Shokoji T, Rahman M, Yao L, Nagai Y, Fujisawa Y, Miyatake A, Abe Y (2005) Cardiac oxidative stress in acute and chronic isoproterenol-infused rats. Cardiovasc Res 65:230–238
Zhang Y, Yan J, Chen K, Song Y, Lu Z, Chen M, Han C, Zhang Y (2004) Different roles of alpha1-adrenoceptor subtypes in mediating cardiomyocyte protein synthesis in neonatal rats. Clin Exp Pharmacol Physiol 31:626–633
Zheng M, Zhang SJ, Zhu WZ, Ziman B, Kobilka BK, Xiao RP (2000) Beta 2-adrenergic receptor-induced p38 MAPK activation is mediated by protein kinase A rather than by Gi or gbeta gamma in adult mouse cardiomyocytes. J Biol Chem 275:40635–40640
Zou Y, Komuro I, Yamazaki T, Kudoh S, Uozumi H, Kadowaki T, Yazaki Y (1999) Both Gs and Gi proteins are critically involved in isoproterenol-induced cardiomyocyte hypertrophy. J Biol Chem 274:9760–9770
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salameh, A., Dhein, S. Adrenergic control of cardiac gap junction function and expression. Naunyn-Schmied Arch Pharmacol 383, 331–346 (2011). https://doi.org/10.1007/s00210-011-0603-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-011-0603-4