Abstract
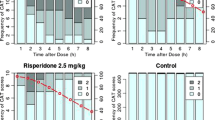
The predictive validity of catalepsy as a rodent model for detecting the extrapyramidal side effects (EPS) of antipsychotic drugs was recently questioned when the novel antipsychotic savoxepine produced little catalepsy in rodents while producing significant EPS in schizophrenic patients. Because catalepsy is viewed as an important model for predicting EPS, we decided to re-evaluate the effects of savoxepine. Savoxepine, clozapine, haloperidol, olanzapine, ORG 5222, raclopride, and risperidone were examined in two tests for catalepsy (grid and bar tests) in male Sprague-Dawley rats. The ability to antagonize amphetamine-induced hypermotility was also examined, since this measure is believed to predict clinical efficacy. With the exception of clozapine, all drugs produced dose-dependent catalepsy in both tests. For each drug, the minimum effective dose for producing catalepsy was greater than or equal to the ED50 for antagonizing amphetamine-induced hyperactivity (defined as the dose producing a 50% reduction in hyperactivity). Clozapine resulted in the widest separation of effective doses in the catalepsy and activity models. Raclopride produced the next largest separation while the remaining drugs resulted in only a one-or two-fold dose separation between the two behavioral tests. The results with haloperidol and clozapine are consistent with the clinical effects of these drugs (severe versus mild EPS). The ratios of effective doses in catalepsy and activity for the remaining novel drugs are also consistent with preliminary clinical findings indicating some EPS with each of these compounds. Thus, catalepsy remains a suitable rodent model for detecting compounds with EPS liability in humans.
Similar content being viewed by others
References
Arnt J (1982) Pharmacological specificity of conditioned avoidance response inhibition in rats: Inhibition by neuroleptics and correlation to dopamine receptor blockade. Acta Pharmacol Toxicol 51:321–329
Arnt J, Christensen AV (1981) Differential reversal by scopolamine and THIP of the antistereotypic and cataleptic effects of neuroleptics. Eur J Pharmacol 69:107–111
Baldessarini RJ (1990) Drugs and the treatment of psychiatric disorders. In: Goodman Gilman A, Rall TW, Nies AS, Taylor P (eds) Goodman and Gilman's the pharmacological basis of therapeutics. Pergamon Press, New York, pp 383–435
Bischoff S (1992) Towards the understanding of the neuroanatomical substrate of schizophrenia: The contribution of savoxepine. In: Meltzer HY (ed) Novel antipsychotic drugs. Raven Press, New York, pp 117–134
Broekkamp CLE, DeGraaf JS, van Delft AML (1990) Behavioral pharmacology of trans-5-chloro-2-methyl-2,3,3a,12b,tetrahydro-1H-dibenz[2, 3: 6, 7]oxepino-[4, 5-c]pyrrolidine maleate, a compound interacting with dopaminergic and serotonergic receptors. Arzneimittelforschung 40:544–549
Bugarski-Kirola D, Bokonjic D, Rosic N, Paunovic VR (1994) Behavioral effects of savoxepine in rats. Neuropsychopharmacology 10:110S
Casey DE (1989) Clozapine: neuroleptic-induced EPS and tardive dyskinesia. Psychopharmacology 99:S47-S53
Chouinard G, Jones B, Remington G, Bloom D, Addington D, MacEwan GW, LaBelle A, Beauclair L, Arnott W (1993) A Canadian multicenter placebo-controlled study of fixed doses of risperidone and haloperidol in the treatment of chronic schizophrenic patients. J Clin Psychopharmacol 13:25–40
Costall B, Naylor RJ (1973) Neuroleptic and non-neuroleptic catalepsy. Arzneimittelforschung 23:674–683
Farde L, Nordstrom A, Wiesel F, Pauli S, Halldin C, Sedvall G (1992) Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Arch Gen Psychiatry 49:538–544
Janssen PAJ, Niemegeers CJE, Awouters F, Schellekens KHL, Megens AHP, Meert TF (1988) Pharmacology of risperidone (R 64 766), a new antipsychotic with serotonin-S2 and dopamine-D2 antagonistic properties. J Pharmacol Exp Ther 244:685–693
Krupp P, Barnes P (1992) Clozapine-associated agranulocytosis: risk and aetiology. Br J Psychiatry [Suppl] 17:38–40
Marder SR, Meibach RC (1994) Risperidone in the treatment of schizophrenia. Am J Psychiatry 151:825–835
McCreadie RG (1992) A double-blind comparison of raclopride and haloperidol in the acute phase of schizophrenia. Acta Psychiatr Scand 86:391–398
McInerney SC, Hoffman DC, Donovan H, Cornfield RJ, Meade RJ, Cassella JV, Gallager DW (1994) Effects of antipsychotics on the pentylenetetrazole (PTZ) seizure threshold relative to in vivo potency may be predictive of clinical seizure liability. Soc Neurosci Abstr 20:1772
Moller HJ, Kissling W, Dietzfelbinger T, Stoll KD, Wendt G (1989) Efficacy and tolerability of a new antipsychotic compound (savoxepine): results of a pilot study. Pharmacopsychiatry 22:38–41
Moore NA, Tye NC, Axton MS, Risius FC (1992) The behavioral pharmacology of olanzapine, a novel “atypical” antipsychotic agent. J Pharmacol Exp Ther 262:545–551
Morelli M, DiChiara G (1985) Catalepsy induced by SCH 23390 in rats. Eur J Pharmacol 117:179–185
Ogren SO, Hall H, Kohler C, Magnusson O, Sjostrand S (1986) The selective dopamine D2 receptor antagonist raclopride discriminates between dopamine-mediated motor functions. Psychopharmacology 90:287–294
Sanberg PR, Bunsey MD, Giordano M, Norman AB (1988) The catalepsy test: its ups and downs. Behav Neurosci 102:748–759
Seeman P (1992) Dopamine receptor sequences: therapeutic levels of neuroleptics occupy D2 receptors, clozapine occupies D4. Neuropsychopharmacology 7:261–284
Sitsen JMA, de Vries MCV (1992) ORG 5222: Preliminary clinical results. In: Meltzer HY (ed) Novel antipsychotic drugs. Raven Press, New York, pp 15–18
Waldmeier PC, Bischoff S, Bittiger H, Hauser K, Vassout A, Delini-Stula A, Haeusler A, Schenkel L, Storni A (1986) Pharmacological profiles of four new tetracyclic dopamine antagonists maroxepine, citatepine, eresepine, and cipazoxapine. Pharmacopsychiatry 19:316–317
Wetzel H, Wiedemann K, Holsboer F, Benkert O (1991) Savoxepine: invalidation of an “atypical neuroleptic response pattern predicted by animal models in an open clinical trial with schizophrenic patients. Psychopharmacology 103:280–283
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hoffman, D.C., Donovan, H. Catalepsy as a rodent model for detecting antipsychotic drugs with extrapyramidal side effect liability. Psychopharmacology 120, 128–133 (1995). https://doi.org/10.1007/BF02246184
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02246184