Abstract
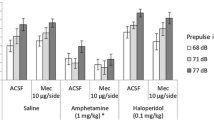
Prepulse inhibition (PPI) is the decrease in a startle response that occurs when the startling stimulus is preceded by a weaker stimulus or “prepulse”. Schizophrenic patients exhibit abnormally low levels of PPI when the prepulse precedes the startle stimulus by less than 500 ms. A similar deficit in sensorimotor gating can be demonstrated in rats after stimulation of D2 dopamine (DA) receptors by systemic administration of DA agonists or by infusion of DA directly into the nucleus accumbens. We now demonstrate that carbachol infusion into the dentate gyrus of the hippocampal formation disrupts PPI in the rat. This disruption of sensorimotor gating occurs when the startling stimulus is either acoustic or tactile. Carbachol infusion into the neocortex has no effect on PPI. While pretreatment with the D2 DA receptor antagonist spiperone reverses the disruption of PPI caused by systemic administration of apomorphine, this pretreatment fails to reverse the disruption of PPI induced by carbachol infusion into the hippocampus. These results demonstrate that pharmacologic stimulation of the hippocampus disrupts sensorimotor gating in the rat by a mechanism distinct from that of DA agonists. Prepulse inhibition of the startle reflex is an animal model in which pharmacologic stimulation of the hippocampus mimics the deficits in sensorimotor gating observed in schizophrenic patients.
Similar content being viewed by others
References
Bickford-Wimer PC, Nagamoto H, Johnson R, Adler LE, Egan M, Rose GM, Freedman R (1990) Auditory sensory gating in hippocampal neurons: a model system in the rat. Biol Psychiatry 27:183–192
Bogerts B, Meertz E, Schonfeld-Bausch R (1985) Basal ganglia and limbic system pathology in schizophrenia. Arch. Gen Psychiatry 42:784–791
Braff DL, Geyer MA (1990) Sensorimotor gating and schizophrenia. Arch Gen Psychiatry 47:181–188
Braff DL, Grillon C, Geyer MA (1991) Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry (in press)
Braff DL, Stone C, Callaway E, Geyer MA, Glick ID, Bali L (1978) Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology 15:339–343
Brown K, Colter N, Corsellis JA, et al. (1986) Postmortem evidence of structural brain changes in schizophrenia. Arch Gen Psychiatry 43:36–42
Creese I, Burt DR, Snyder SH (1975) Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science 192:481–483
Davis M (1984) The mammalian startle response. In: Eaton RC (ed) Neural mechanisms of startle behavior. Plenum Pess, New York
Emerich DF, Walsh TJ (1990) Hyperactivity following intradentate injection of colchicine: a role for dopamine systems in the nucleus accumbens. Pharmacol Biochem Behav 37:149–154
Falkai P, Bogerts B (1986) Cell loss in the hippocampus of schizophrenics. Eur Arch Psychiatry Neurol Sci 236:154–161
Falkai P, Bogerts B, Rozumik M (1988) Limbic pathology in schizophrenia: The entorhinal region — a morphometric study. Biol Psychiatry 24:515–521
Farmery SM, Owen F, Poulter M, et al. (1985) Reduced high affinity cholecystokinin binding in hippocampus and frontal cortex of schizophrenic patients. Life Sci 36:473–477
Ferrier IN, Roberts GW, Crow TJ, Johnstone EC, Owens DG, Lee YC, O'Shaughnessy D, Adrian TE, Polak JM, Bloom SR (1983) Reduced cholecystokinin-like and somatostatin-like immunoreactivity in limbic lobe is associated with negative symptoms in schizophrenia. Life Sci 33:475–482
Flicker C, Geyer MA (1982) Behavior during hippocampal microinfusions: II. Muscarinic locomotor activation. Brain Res Rev 4:105–127
Geyer MA, Swerdlow NR, Mansbach RS, Braff DL (1990) Startle response models of sensorimotor gating and habituation deficits in schizophrenia. Brain Res Bull 25:485–498
Graham FK (1975) The more or less starting effect of weak prestimuli. Psychophysiology 12:238–248
Handelmann GE, Olton DS (1981) Spatial memory following damage to hippocampal CA3 cells with kainic acid: impairment and recovery with postoperative training. Brain Res 217:41–58
Handley SL, Thomas KV (1979) Potentiation of startle response byd-andl-amphetamine: the possible involvement of pre- and postsynaptic alpha-adrenoceptors and other transmitter systems in the modulation of the tactile startle response. Psychopharmacology 64:105–112
Heath RG (1954) Studies in schizophrenia. Harvard University Press, Cambridge, MA
Hoffman HS, Searle JL (1968) Acoustic and temporal factors in the evocation of startle. J Acoust Soc Am 43:269–282
Jakob H, Beckmann H (1986) Prenatal development disturbances in the limbic allocortex in schizophrenics. J Neural Transm 65:303–326
Jeste DV, Lohr JB (1989) Hippocampal pathologic findings in schizophrenia. Arch Gen Psychiatry 46:1019–1024
Kerwin RW, Patel S, Meldrum BS, Czudek C, Reynolds GP (1988) Asymmetrical loss of glutamate receptor subtype in left hippocampus in schizophrenia. Lancet 1[8585]: 583–584
Luchins DJ (1990) A possible role of hippocampal dysfunction in schizophrenic symptomatology. Biol Psychiatry 28:87–91
Mansbach RS, Geyer MA (1990) Effects of phencyclidine and phencyclidine biologs on sensorimotor gating in the rat. Neurophsychopharmacology 2:299–308
Mansbach RS, Geyer MA, Braff DL (1988) Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology 94:507–514
Mogenson GJ, Nielson M (1984) A study of the contribution of hippocampal-accumbens-subpallidal projections to locomotor activity. Behav Neural Biol 42:38–51
Newton MW, Crosland RD, Jenden DJ (1985) Effects of dietary administration of cholinergic false precursor N-amino-N,N-dimethylaminoethanol on behavior and cholinergic parameters in rats. Brain Res 373:197–204
Overstreet DH (1977) Pharmacological approaches to habituation of the acoustic startle response in rats. Physiol Phsychol 5:230–238
Peng RY, Mansbach RS, Braff DL, Geyer MA (1990) A D2 dopamine receptor agonist disrupts sensorimotor gating in rats: Implications for dopaminergic abnormalities in schizophrenia. Neuropsychopharmacology 3:211–218
Reynolds GP (1983) Increase concentrations and lateral asymmetry of amygdala dopamine in schizophrenia. Nature 305:527–529
Reynolds GP (1987) Post-mortem neurochemical studies in schizophrenia. In: Haefner H, Gattaz WF, Janzarik W (eds) Search for the Causes of Schizophrenia Springer, Berlin Heidelberg New York
Roberts GW (1989) Brain development and CCK systems in schizophrenia: a working hypothesis. In: Weller MPO (ed) Advances in biological psychiatry. Libbey, London
Suddath RL, Cassanove MF, Goldberg TE, Daniel DG, Kelsoe JR, Weinberger DR (1989) Temporal lobe pathology in schizophrenia: a quantitative magnetic resonance imaging study. Am J Psychiatry 146:464–472
Swerdlow NR, Braff DL, Geyer MA, Koob GF (1986) Central dopamine hyperactivity in rats mimics abnormal sensory gating of the acoustic startle response in schizophrenics. Biol Psychiatry 21:23–33
Swerdlow NR, Mansbach RS, Geyer MA, Pulvirenti L, Koob GF, Braff DL (1990b) Amphetamine disruption of prepulse inhibition of acoustic startle is reversed by nucleus accumbens denervation. Psychopharmacology 100:413–416
Swerdlow NR, Masten VL, Braff DL, Geyer MA (1990c) Schizophrenic-like sensorimotor gating abnormalities in rats following dopamine infusion into the nucleus accumbens. Psychopharmacology 101:414–420
Swerdlow NR, Keith VA, Braff DL, Geyer MA (1991) The effects of spiperone, raclopride, SCH 23390 and clozapine on apomorphine-inhibition of sensorimotor gating of the acoustic startle response in the rat. J Pharmacol Exp Ther 256:530–536
Walsh TJ, Tilson HA, DeHaven DL, Mailman RB, Fisher A. Hanin I (1984) AF64A, a cholinergic neurotoxin, selectively depletes acetylcholine in hippocampus and cortex, and produces long-term passive avoidance and radial-arm maze deficits in the rat. Brain Res 321:91–102
Walsh TJ, Schulz DW, Tilson HA, Schmechel DE (1986) Colchicine-induced granule cell loss in rat hippocampus: selective behavioural and histological alterations. Brain Res 398:23–36
Warburton DM, Groves PM (1969) The effects of scopolamine on habituation of acoustic startle in rats. Commun Behav Biol 3:289–293
Williams JM, Hamilton LW, Carlton PL (1974) Pharmacological and anatomical dissociation of two types of habituation. J Comp Physiol Psychol 87:724–732
Wu MF, Jenden DJ, Fairchild MD, Szymusiak RS, McGinty DJ, Siegel JM (1990a) Modulation of the acoustic startle reflex after dietary false choline precursor NADE. Soc Neurosci Abstr 16[1]:727
Wu JC, Segal BV Jr, Haier RJ, Buchsbaum MS (1990b) Testing the Swerdlow/Koob model of schizophrenia pathophysiology using positron emission tomography. Behav Brain Sci 13[1]:169–170
Zheng S, Berman HA, Geyer MA (1983) Behavior during hippocampal microinfusions: anticholinesterase-induced locomotor activation. Behav Brain Res 9:295–304
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Caine, S.B., Geyer, M.A. & Swerdlow, N.R. Carbachol infusion into the dentate gyrus disrupts sensorimotor gating of startle in the rat. Psychopharmacology 105, 347–354 (1991). https://doi.org/10.1007/BF02244429
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02244429