Abstract
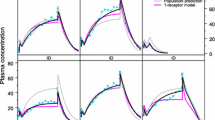
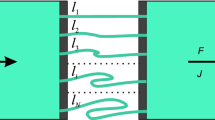
A dispersion model of hepatic elimination, based on the residence time distribution of blood elements within the liver, is presented. The general rate equations appropriate for describing the hepatic output concentration of a tracer solute are derived. Particular consideration is given to events following a bolus input dose of a tracer. The model is shown to be compatible with the known hepatic architecture and hepatic physiology. The model has been fitted to hepatic outflow data for red blood cells, albumin, and other noneliminated solutes. The experimental data suggest a high degree of dispersion of blood elements within the liver. The model has also been used to evaluate the effects of changes in enzyme activity, hepatic cell permeability, blood flow, and protein binding on the outflow concentration vs. time profiles of solutes.
Similar content being viewed by others
References
M. Rowland, L. Z. Benet, and G. G. Graham. Clearance concepts in pharmacokinetics.J. Pharmacokin. Biopharm. 1:123–136 (1977).
G. R. Wilkinson and D. G. Shand. Commentary. A physiological approach to hepatic drug clearance.Clin. Pharmacol. Ther. 18:377–390 (1975).
D. L. Miller, C. S. Zanolli, and J. J. Gumucio. Quantitative morphology of the sinusoids of the hepatic acinus: Quantimet analysis of rat liver.Gastroenterology 76:965–969 (1979).
A. I. Koo, I. Y. S. Liang, and K. K. Cheng. The terminal hepatic microcirculation in the rat.Q. J. Exp. Physiol,60:261–266 (1975).
J. J. Gumucio and D. L. Miller. Function implications of liver cell heterogeneity.Gastroenterology 80:393–403 (1981).
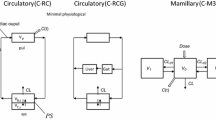
K. S. Pang and M. Rowland. Hepatic clearance of drugs. 1. Theoretical considerations of a “well-stirred” model and a “parallel tube” model. Influence of hepatic blood flow, plasma and blood cell binding and hepatocellular enzymatic activity on hepatic drug clearance.J. Pharmacokin. Biopharm. 5:625–653 (1977).
O. Levenspiel.Chemical Reaction Engineering, Wiley, New York, 1972, pp. 253–315.
L. Bass, P. J. Robinson, and A. J. Bracken. Hepatic elimination of flow substances: The distributed model.J. Theor. Biol. 72:161–184 (1978).
E. L. Forker and B. Luxon. Hepatic transport kinetics and plasma disappearance curves: Distributed modelling versus conventional approach.Am. J. Physiol. 235:E648-E660 (1978).
K. S. Pang and M. Rowland. Hepatic clearance of drugs. 2. Experimental evidence for acceptance of the “well-stirred” model over the “parallel-tube” model using lidocaine in the perfused rat liverin situ preparation.J. Pharmacokin. Biopharm. 5:655–683 (1977).
S. Keiding and E. Chiarantini. Effect of sinusoidal perfusion on galactose elimination in perfused rat liver.J. Pharmacol. Exp. Ther. 205:465–470 (1978).
A. B. Ahmad, P. N. Bennett, and M. Rowland. Models of hepatic drug clearance: Discrimination between the “well-stirred” and “parallel-tube” models.J. Pharm. Pharmacol. 35:219–224 (1983).
S. Keiding and E. Steiness. Flow dependence of propranolol elimination in perfused rat liver.J. Pharmacol. Exp. Ther. 230:474–477 (1984).
D. B. Jones, D. J. Morgan, G. W. Mihaly, L. K. Webster, and R. A. Smallwood. Discrimination between the venous equilibrium and sinusoidal models of hepatic drug elimination in the isolated perfused rat liver by perturbation of propranolol protein binding.J. Pharmacol. Exp. Ther. 229:522–526 (1984).
M. Rowland, D. Leitch, G. Fleming, and B. Smith. Protein binding and hepatic clearance: Discrimination between models of hepatic clearance with diazepam, a drug of high intrinsic clearance, in the isolated perfused rat liver preparation.J. Pharmacokin. Biopharm. 12:129–147 (1984).
L. Bass. Models of hepatic elimination.J. Pharm. Sci. 72:1229 (1983).
D. J. Morgan. Models of hepatic elimination: A response.J. Pharm. Sci. 72:1230 (1983).
E. L. Forker and B. A. Luxon. Albumin binding and hepatic uptake: The importance of model selection.J. Pharm. Sci. 72:1232–1233 (1983).
W. A. Colburn. Albumin binding and hepatic uptake: The importance of model selection- A response.J. Pharm. Sci. 72:1233 (1983).
M. S. Roberts and M. Rowland. A dispersion model of hepatic elimination: 2. Steady-state considerations-Influence of hepatic blood flow, binding within blood, and hepatocellular enzyme activity.J. Pharmacokin. Biopharm. 14:261–268 (1986).
M. S. Roberts and M. Rowland. Hepatic elimination: A dispersion model.J. Pharm. Sci. 74:585–587 (1985).
O. Levenspiel and W. K. Smith. Notes on the diffusion-type model for longitudinal mixing of fluids in flow.Chem. Eng. Sci. 6:227–233 (1957).
G. F. Froment and K. B. Bischoff.Chemical Reactor Analysis and Design, Wiley, New York, 1979.
L. Bass, S. Keiding, K. Winkler, and N. Tygstrup. Enzymatic elimination of substances flowing through the intact liver.J. Theor. Biol. 61:393–409 (1976).
P. V. Dankwerts. Continuous flow systems. Distribution of residence times.Chem. Eng. Sci. 2:1–18 (1952).
C. Y. Wen and L. T. Fan.Models for Flow Systems and Chemical Reactors, Marcel Dekker, New York, 1975.
E. T. Van der Laan. Notes on the diffusion-type model for the longitudinal mixing of flow.Chem. Eng. Sci. 7:187–191 (1957).
J. C. Jaeger and G. N. Newstead.An Introduction to the Laplace Transform, Methuen, London, 1968.
C. Y. Wen and L. T. Fan.Models for Flow Systems and Chemical Reactors, Marcel Dekker, New York, 1975, p. 134.
W. Perl and F. P. Chinard. A correction-diffusion model of indicator transport through an organ. Circ. Res.22:273–298 (1967).
H. Brenner. Diffusion model of longitudinal mixing in beds of finite length. Numerical values.Chem. Eng. Sci. 17:229–243 (1962).
R. V. Churchill.Operational Mathematics, 2nd ed., McGraw-Hill, New York, 1958, p. 15.
J. F. Wehner and R. H. Wilhelm. Boundary conditions of flow reactor.Chem. Eng. Sci. 6:89–93 (1956).
J. R. Pearson. A note on the “Dankwerts” boundary conditions for continuous flow reaction.Chem. Eng. Sci. 10:281–285 (1959).
C. W. Sheppard.Basic Principles of the Tracer Method, Wiley, New York, 1962, pp. 251–253.
M. Weiss. Moments of physiological transit time distribution and the time course of drug disposition in the body.J. Math. Biol. 15:305–318 (1982).
C. A. Goresky. A linear model for determining liver sinusoidal and extravascular volume.Am. J. Physiol. 204:626–640 (1963).
P. Veng-Pederson. Curve fitting and modelling in pharmacokinetics and some practical experience with NONLIN and a new program FUNFIT.J. Pharmacokin. Biopharm. 5:513–531 (1977).
C. A. Goresky. Kinetic interpretation of hepatic multiple indicator dilution studies.Am. J. Physiol. 245:G1-G12 (1983).
C. A. Goresky, G. G. Bach, and B. E. Nadeau. On the uptake of materials by the intact liver: The transport and net removal of galactose.J. Clin. Invest. 52:991–1009 (1973).
A. L. Jones, G. T. Hradek, R. H. Renston, K. Y. Wong, G. Karlagancs, and G. Paumgartner. Autoradiographic evidence for hepatic lobular concentration gradient of bile and derivative.Am. J. Physiol. 238:G233-G237 (1980).
J. J. Gumucio, D. L. Miller, M. D. Krauss, and C. C. Zanolli. Transport of fluorescent compounds into hepatocytes and the resultant zonal labelling of the hepatic acinus in the rat.Gastroenterology 80:639–646 (1981).
J. J. Gumucio. Functional and anatomic heterogeneity in the liver cinus: Impact on transport.Am. J. Physiol. 245:G578-G582 (1983).
J. W. Grisham and W. Nopanitaya. Scanning electron microscopy of casts of hepatic microvessels: Review of methods and results. In W. W. Lautt (ed.),Hepatic Circulation in Health and Disease, Raven Press, New York, 1981, pp. 87–109.
A. M. Rappaport. The acinus-microvascular unit of the liver. In W. W. Lautt (ed.),Hepatic Circulation in Health and Disease, Raven Press, New York, 1981, pp. 175–192.
H. Kramers and K. R. Westerterp.Elements of Chemical Reaction Design and Operation, Academic Press, New York, 1963, pp. 70–73.
C. Y. Choi and D. D. Perlmutter. A unified treatment of inlet boundary condition for dispersion flow models.Chem. Eng. Sci. 31:250–252 (1976).
L. G. Gibilaro. On the residence time distribution for systems with open boundaries.Chem. Eng. Sci. 33:487–492 (1978).
C. A. Goresky, E. R. Gordon, and G. G. Bach. Uptake of monohydric alcohols by liver: Demonstration of a shared enzyme space.Am. J. Physiol. 244:G198-G214 (1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Roberts, M.S., Rowland, M. A dispersion model of hepatic elimination: 1. Formulation of the model and bolus considerations. Journal of Pharmacokinetics and Biopharmaceutics 14, 227–260 (1986). https://doi.org/10.1007/BF01106706
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01106706