Summary
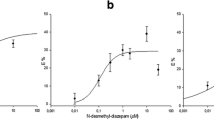
The effects of barbiturates on radioligand binding to inhibitory R i adenosine receptors of rat brain membranes were investigated. Binding of the adenosine receptor agonist (−)N6-phenylisopropyl[3H]adenosine and the antagonist 1,3-diethyl-8-[3H]phenylxanthine was inhibited by several barbiturates. This inhibition was concentration-dependent and occurred in the range of pharmacologically effective concentrations. Pentobarbital was the most potent of the barbiturates tested with a K i of 92 μmol/l. The (+)isomers of hexobarbital and mephobarbital were more potent than the respective (−)isomers. Barbituric acid itself did not displace either radioligand in concentrations up to 1 mmol/l. The inhibitory effect of pentobarbital was reversed by a single wash of membranes preincubated with the barbiturate. The presence of pentobarbital caused a decrease of the affinity of the receptor for the antagonist radioligand but did not alter the number of binding sites, suggesting a competitive antagonism. The effects of pentobarbital on radioligand binding to the receptor were not changed by the presence of picrotoxinin nor by the absence of chloride ions. This indicates that they are not mediated via the picrotoxinin binding site. The barbiturates could not be classified as either agonists or antagonists at the R i adenosine receptor. The presence of GTP did not influence the inhibition of radioligand binding by pentobarbital; this is also observed for antagonists, whereas the affinity of agonists is markedly reduced by GTP. Binding of antagonists to the receptor is enthalpy-driven; the interaction of pentobarbital with the receptor was entropy-driven and the same was true for agonists. Thus, the interaction of pentobarbital with R i adenosine receptors of rat brain membranes differs from that of both adenosine agonists and antagonists. Our data suggest that R i adenosine receptors may be involved in the mediation of the effects of barbiturates.
Similar content being viewed by others
References
Bruns RF, Daly JW, Snyder SH (1980) Adenosine receptors in brain membranes: Binding of N6-cyclohexyl[3H]adenosine and 1,3-diethyl-8-[3H]phenylxanthine. Proc Natl Acad Sci USA 77:5547–5551
Büch H, Grund W, Buzello W, Rummel W (1969) Narkotische Wirksamkeit und Gewebsverteilung der optischen Antipoden des Pentobarbitals bei der Ratte. Biochem Pharmacol 18:1005–1009
Burnstock G, Brown CM (1981) An introduction to purinergic receptors. In: Burnstock G (ed) Purinergic receptors. Chapman and Hall, London, pp 1–45
Cooper DMF, Londos C, Rodbell M (1980) Adenosine receptormediated inhibition of rat cerebral cortical adenylate cyclase by a GTP-dependent process. Mol Pharmacol 18:598–601
De Lean A, Hancock AA, Lefkowitz RJ (1982) Validation and statistical analysis of a computer modeling method for quantitative analysis of radioligand binding data for mixtures of pharmacological receptor subtypes. Mol Pharmacol 21:5–16
Ebersolt C, Prémont J, Bockaert J (1983) Inhibition of brain adenylate cyclase by A1 adenosine receptors: Pharmacological characteristics and locations. Brain Res 267:123–129
Goodman RR, Cooper MJ, Gavish M, Snyder SH (1982) Guanine nucleotide and cation regulation of the binding of [3H]cyclohexyladenosine and [3H]diethylphenylxanthine to adenosine A1 receptors in brain membranes. Mol Pharmacol 21:329–335
Hirsch MD (1983) Barbiturate competition for TRH receptors in mouse brain: neuromodulation of anesthesia. Peptides 4:255–260
Ho IK, Harris RA (1981) Mechanism of action of barbiturates. Ann Rev Pharmacol Toxicol 21:83–111
Houslay MD, Dipple I, Gordon LM (1981) Phenobarbital selectively modulates the glucagon-stimulated activity of adenylate cyclase by depressing the lipid phase separation occurring in the outer half of the bilayer of liver plasma membranes. Biochem J 197:675–681
Jackisch R, Strittmatter H, Fehr R, Hertting G (1983) Modulation of hippocampal noradrenaline and acetylcholine release by endogenous adenosine. Naunyn-Schmiedeberg's Arch Pharmacol [Suppl] 324:R77
Murphy KMM, Snyder SH (1982) Heterogeneity of adenosine A1 receptor binding in brain tissue. Mol Pharmacol 22:250–257
Lohse MJ, Lenschow V, Schwabe U (1983) Interaction of barbiturates with adenosine receptors in rat brain. Naunyn-Schmiedeberg's Arch Pharmacol [Suppl] 324:R78
Londos C, Cooper DMF, Wolff J (1980) Subclasses of external adenosine receptors. Proc Natl Acad Sci USA 77:2551–2554
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Olsen RW (1982) Drug interactions at the GABA receptorionophore complex. Ann Rev Pharmacol Toxicol 22:245–277
Phillis JW, Wu PH (1981) The role of adenosine and its nucleotides in central synaptic transmission. Prog Neurobiol 16:187–239
Phillis JW, Wu PH (1983) The role of adenosine in central neuromodulation. In: Berne RM, Rall TW, Rubio R (eds) Regulatory function of adenosine. Martinus Nijhoff Publishers, The Hague Boston London, pp 419–437
Prémont J, Perez M, Bockaert J (1977) Adenosine sensitive adenylate cyclase in rat striatal homogenates and its relationship to dopamine- and Ca2+-sensitive adenylate cyclases. Mol Pharmacol 13:662–670
Schubert P, Lee K, Reddington M, Kreutzberg G (1983) Synaptic modulation by adenosine: Electrophysiological and biochemical characteristics. In: Berne RM, Rall TW, Rubio R (eds) Regulatory function of adenosine. Martinus Nijhoff Publishers, The Hague Boston London, pp 439–454
Schwabe U, Trost T (1980) Characterization of adenosine receptors in rat brain by (−)[3H]N6-phenylisopropyladenosine. Naunyn-Schmiedeberg's Arch Pharmacol 313:179–187
Snyder SH, Katims JJ, Annau Z, Bruns RF, Daly JW (1981) Adenosine receptors and behavioral actions of methylxanthines. Proc Natl Acad Sci USA 78:3260–3264
Ticku MK, Olsen RW (1978) Interaction of barbiturates with dihydropicrotoxinin binding sites related to the GABA receptor-ionophore system. Life Sci 22:1643–1652
Van Calker D, Müller M, Hamprecht B (1978) Adenosine inhibits the accumulation of cyclic AMP in cultured brain cells. Nature 276:839–841
Walström G (1966) Differences in the anaesthetic properties between the optical antipodes of hexobarbital in the rat. Life Sci 5:1781–1790
Weiland GA, Minnemann KP, Molinoff PB (1979) Fundamental difference between the molecular interactions of agonists and antagonists with the β-adrenergic receptor. Nature 281: 114–117
Whittaker VP (1969) The synaptosome. In: Lajtha A (ed) Handbook of neurochemistry, vol 2. Plenum Press, New York, pp 327–364
Williams M, Risley EA (1980) Biochemical characterization of putative central purinergic receptors by using 2-chloro[3H]-adenosine, a stable analog of adenosine. Proc Natl Acad Sci USA 77:6892–6896
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lohse, M.J., Lenschow, V. & Schwabe, U. Interaction of barbiturates with adenosine receptors in rat brain. Naunyn-Schmiedeberg's Arch. Pharmacol. 326, 69–74 (1984). https://doi.org/10.1007/BF00518781
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00518781