Article Text
Abstract
Background: The loss of cystic fibrosis transmembrane conductance regulator (CFTR) mediated chloride conductance does not fully explain the diverse pathologies evident in patients with cystic fibrosis (CF). Bicarbonate (HCO3−) secretion is also impaired in CFTR expressing tissues and CFTR is thought to regulate HCO3− secretion at the apical membrane of epithelial cells. We hypothesised that the epithelial lining fluid (ELF) of patients with CF would be acidified and that this may be worsened during an infective exacerbation due to the increased inflammatory burden.
Methods: pH and nitrite levels in exhaled breath condensate (EBC) from 12 healthy non-smoking controls and 30 patients with CF (11 of whom were in an infective exacerbation) were measured. A further nine patients were studied before and after intravenous antibiotic treatment for an exacerbation of CF.
Results: The pH of EBC was significantly lower in patients with stable CF than in controls (5.88 (0.32) v 6.15 (0.16), p=0.017), and was further reduced in CF patients with an exacerbation (5.32 (0.38), p=0.001) compared with stable CF patients. EBC pH increased significantly following antibiotic treatment from 5.27 (0.42) to 5.71 (0.42), p=0.049). Nitrite levels in EBC were increased in CF patients with an exacerbation compared with control subjects (4.4 (4.0) μm v 1.6 (1.6) μm p=0.047). No correlation was found between EBC pH and nitrite levels.
Conclusions: These findings support the hypothesis that airway acidification occurs in CF. This acidity is in part a function of inflammation as the pH of the EBC of patients increased significantly with treatment of an exacerbation, although not to control levels. Acidic pH of the ELF may play a role in the pathophysiology of CF lung disease and requires further investigation.
- cystic fibrosis
- pH
- breath condensate
- airway inflammation
- CFTR
Statistics from Altmetric.com
Cystic fibrosis (CF) results from mutations in the CF transmembrane conductance regulator (CFTR). Since the identification and cloning of the CFTR gene it has become established that CFTR functions as a cyclic AMP regulated chloride channel on the apical membrane of epithelial cells. However, despite this advance in understanding, the pathogenesis of severe lung disease in patients with CF has been difficult to explain by the loss of chloride conductance alone.1
Although bicarbonate (HCO3−) secretion has been shown to be impaired in CF tissues compared with tissues with normal expression of CFTR, this has so far received little attention.2 Several investigators have shown that CFTR conducts epithelial bicarbonate transport both in cell culture systems and in intact airway.3–10 Choi et al11 found that various CFTR mutations associated with pancreatic insufficiency have greatly reduced HCO3− secretion in vitro compared with mutations associated with pancreatic sufficiency. They and others have highlighted the importance of the role of CFTR in HCO3− and fluid transport in relation to pancreatic disease in CF, suggesting that relatively acidic fluid secretion in the pancreas leads to precipitation of mucins, plugging of ductal systems, and alterations in enzyme activity.11–13 In normal airway epithelial cells it is likely that significant amounts of HCO3− cross the apical membrane into the airway lumen and that this process is facilitated by CFTR.3–7 Given the small volume of airway epithelial lining fluid (ELF), such a failure of this HCO3− transport mechanism together with the chronic neutrophilic inflammation in the CF airway could cause the ELF to become acidified. This could have significant consequences for the airway defences since ciliary function, mucus viscosity, bacterial binding, and defensins are all adversely affected in more acidic environments.14–19 Attempts to estimate airway pH in vivo have usually involved invasive techniques with the introduction of pH probes either bronchoscopically via endotracheal tubes or via tracheostomies, usually to the proximal airway.20–22
We have investigated whether CF airways might be acidified compared with healthy subjects using the non-invasive technique of measuring the pH of exhaled breath condensate in an attempt to avoid sampling difficulties associated with ELF. We also examined the relationship between pH and levels of breath condensate nitrite, an existing inflammatory marker.23
METHODS
Subjects
Thirty adult patients with CF (20 men) of mean (SD) age 24 (4.5) years with known genotype attending the Scottish Adult Cystic Fibrosis Service, Edinburgh were recruited. Eleven were judged clinically to be in an infective exacerbation, prospectively defined as treatment with intravenous antibiotics for any four of the following 10 signs or symptoms: change in sputum; new or increased haemoptysis; increased cough; increased dyspnoea; malaise or lethargy; temperature above 38°C; anorexia or weight loss; change in physical examination of the chest; decrease in pulmonary function by 10% or more from a previously recorded value; or radiographic changes of pulmonary infection. The remaining 19 were clinically stable—that is, they were not receiving antibiotics or a consistent regimen of maintenance antibiotics during the 14 days before collection of breath condensate and had no signs of an exacerbation. A further nine patients with CF presenting with an exacerbation of respiratory symptoms were followed prospectively to completion of treatment (defined according to the above criteria). Twelve healthy non-smoking subjects (seven men) of mean (SD) age 33 (8) years acted as controls.
The pH of the breath condensate was measured on the day of presentation (day 1) and at the completion of intravenous antibiotics (day 14). The mean (SD) absolute forced expiratory volume in 1 second (FEV1) of the CF patients was 1.96 (0.9) l (mean % predicted 51%, range 16–104%).
Collection of breath condensate
Breath condensate was collected using a previously validated technique by subjects exhaling repeatedly from total lung capacity through a 1.5 m Teflon perfluoroalkoxy (PFA) tube of 0.5 cm internal diameter immersed in ice. This method avoids both nasal and salivary contamination and yields approximately 1–2 ml of condensate after 10 minutes.23
pH, nitrite, and peak alveolar CO2 measurements
The pH of condensate samples was measured within 5 minutes of collection (Corning pH Microelectrode, Corning, NY, USA). The system was recalibrated before each analysis. The nitrite concentration in exhaled condensate was determined by a colorimetric assay based on the Greiss reaction as described previously.23 Peak alveolar CO2 measurements (Infrared Absorption Analyzer, Logan-Sinclair Research, Kent, UK) were measured immediately before collection of breath condensate.
The intrasubject reproducibility of condensate pH on different days (2–3 days each) was measured in eight normal subjects and eight stable CF patients.
Statistical analyses
Comparisons between groups were made using the unpaired t test if data were normally distributed, otherwise the Mann-Whitney rank sum test was applied. The correlations between breath condensate pH, nitrite, and FEV1 in patients with CF were measured using Pearson product moment correlation. Statistical calculations were made using SigmaStat 2.03 (SPSS Science Software, Birmingham, UK).
RESULTS
Exhaled condensate pH
The pH of the exhaled breath condensate was lower in all patients with CF than in controls (mean (SD) 5.67 (0.45) v 6.15 (0.16), p=0.002; fig 1). The pH of the airway condensate of stable CF patients (5.88 (0.32)) and of CF patients with an infective exacerbation (5.32 (0.38)) was lower than control subjects (p=0.017 and p=0.001, respectively). The pH of the condensate of CF patients with an exacerbation was significantly lower than that of patients with stable CF (p=0.001).
pH of breath condensate in CF and control groups. Airway condensate pH was acidified in patients with stable CF compared with healthy subjects (mean pH 5.88 (0.32) v 6.15 (0.16), p=0.017). Patients with stable CF had higher condensate pH values than CF patients in exacerbation (5.88 (0.32) v 5.32 (0.38), p=0.001). CF patients with exacerbations had almost one log order lower condensate pH than healthy subjects (p<0.001).
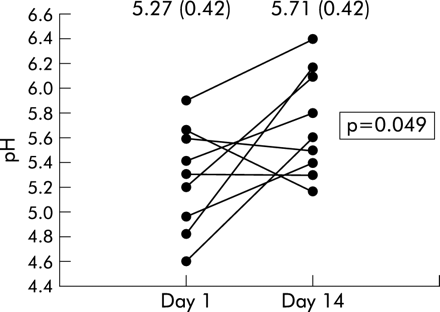
The pH of the exhaled breath condensate of CF patients followed longitudinally through an exacerbation was significantly higher after completion of antibiotic treatment (5.71 (0.42) v 5.27 (0.42), p=0.049; fig 2).
Effect of treatment of exacerbations on breath condensate pH. Values for each patient (n=9) are shown at day 1 of the exacerbation and after 2 weeks of treatment with parenteral antibiotics (day 14). Mean condensate pH after treatment was significantly raised compared with day 1 (5.71 (0.42) v 5.27 (0.42), p=0.049).
There was no correlation between lung function (% predicted FEV1) and exhaled condensate pH in patients with CF (r=0.16, p=0.42). In a group of patients with CF (n=7) in whom arterial gas estimations were performed, the pH of the breath condensate was also unrelated to the arterial CO2 tension (r=–0.15, p=0.75) or to peak alveolar CO2 concentration (r=0.36, n=42).
Intrasubject variation
The mean (SD) difference in condensate pH values between paired serial measurements in normal subjects (n=8) and patients with stable CF (n=8) was 0.09 (0.04) units. The coefficient of repeatability of the test was therefore 0.08 pH units.
Exhaled condensate nitrite
Mean nitrite concentrations in exhaled breath condensate were higher in CF patients with an infective exacerbation than in controls (4.4 (4.0) μM v 1.6 (1.6) μM, p=0.047). There was a trend to lower condensate nitrite levels in stable CF patients compared with CF patients with exacerbations (2.5 (1.7) μM, p=0.096; fig 3). There was no correlation between the condensate pH and nitrite levels in any group (r=–0.15, p=0.44).
Range of breath condensate nitrite levels in stable CF patients and those with exacerbations. Breath condensate nitrite was increased in CF patients with an exacerbation compared with healthy controls (4.4 (4.0) μM v 1.6 (1.6) μM; p=0.047). There was a non-significant trend to higher nitrite levels in patients with stable CF compared with controls (2.5 (1.7) μM; p=0.2).
DISCUSSION
The technique of breath condensate collection represents a new method for non-invasive monitoring of inflammatory lung disease. Markers of inflammation or oxidative stress such as nitrite, hydrogen peroxide, and isoprostane in breath condensate have been advocated for the assessment of the CF airway.23–25
Exhaled breath condensate is composed of condensed water vapour and microdroplets of ELF containing non-volatile solutes and various volatile organic compounds. Although it is probable that the pH of breath condensate is not a direct measurement of ELF pH in situ, it is reasonable to assume that changes in the exhaled breath condensate reflect changes within the ELF. The breath condensate pH values were not an artefact of lung function as there was no correlation between % predicted FEV1 (or absolute FEV1) and condensate pH in the CF group. We also found no correlation between arterial CO2 tension and condensate pH (r=–0.15, p=0.75). Furthermore, peak alveolar CO2 levels were measured immediately before breath condensate pH analysis and no correlation was found (r=0.036, n=42).
pH estimations of ELF in previous studies have largely been derived from direct application of microelectrodes to the proximal tracheal surface in an invasive manner (via tracheostomies or intubation) or from in vitro studies of human airway cell cultures or other mammals and range from 6.2 in the fetal lung to 6.5–7.5 in healthy adults.20–22,26,27 The mean (SD) condensate pH in the healthy subjects reported here (6.15 (0.16)) is lower than those quoted above and is probably explained by the fact that breath condensate is not pure ELF, or perhaps exhaled condensate is a more representative assessment of the whole lung environment, including the distal airways, than that obtained from a localised proximal measurement or an in vitro system.
Biochemical analysis of distal airway ELF in vivo is technically difficult and is usually confounded by the administration of bronchoscopically administered substances (lavage fluid, topical anaesthetics) or by small volumes and thus is difficult to interpret with confidence. Jayaraman et al27 recently found the pH of ELF from intact human airway in vitro to be 6.8–7.0 with an estimated HCO3− concentration of 6–8 mM. However, estimations from in vitro systems may not be directly applicable clinically as the in vivo airway is intermittently exposed to inflammatory stimuli and constantly exposed to environmental particulate matter including pollutants which may contribute to alterations in ELF pH.
Hunt and coworkers reported that breath condensate in acute asthma was acidified compared with healthy adults. This seemed to be a reflection of airway inflammation since the acidity normalised to control values with treatment of the acute asthma.28 However, our findings differed in that the pH of stable CF condensate was significantly more acidic than that of control subjects. It is probable that excessive inflammation can lower the pH of ELF although, if CFTR is responsible at least in part for lumenal HCO3− conductance, then coping mechanisms in CF to compensate for inflammation induced airway acidity may be overwhelmed even during stable disease, unlike the asthmatic airway where normal HCO3− transport should be maintained.
Our results suggest that there is a chronic acidification of the CF airway which may be related to the reduced bicarbonate secretory function of the CF lung leading to reduced buffering capacity and reduced ability to cope with the persistent inflammation. The increase in EBC pH towards control levels following treatment of an exacerbation suggests that the acidity of the CF airway is in part a function of inflammation.
Consistent with previous results, we found that condensate nitrite levels were raised in CF patients with an exacerbation compared with controls. However, there was no correlation between nitrite levels and condensate pH in any group. It is probable that condensate pH and nitrite reflect different processes within the airway, and in patients with CF the condensate pH may be influenced by genotype. Choi et al11 showed that cultured cells expressing CFTR mutations known to be associated with pancreatic sufficiency (class 4–5 mutations such as R117H, A455E) displayed significantly greater bicarbonate conductance than mutations associated with pancreatic insufficiency (class 1–3 mutations, G452X, ΔF508, G551D). This may be difficult to demonstrate in vivo because of varying degrees of airway inflammation even among stable CF patients and the increased complexity of the in vivo airway compared with cell culture.
The pH of airway ELF is of clinical significance because it is known that a more acid pH has a number of detrimental effects on different components of the airway defences. For example, ciliary beat frequency has been shown to be sensitive to alterations in pH below 7.5 in bronchi and below 5.5 in bronchioles.14 Mucus viscosity is increased at lower pH, bacterial binding to mucus is enhanced, and the activity of defensins has been shown to be impaired.15–17,19 Ishizuka and coworkers18 have shown that acid exposure can increase the activity of nuclear factor (NF)-κB in cultured airway epithelial cells and increase the adherence of Streptococcus pneumoniae, possibly mediated by increased expression of platelet activating factor (PAF) receptors induced by NF-κB. Furthermore, it is known that the in vitro bactericidal activity of various antibiotics, particularly aminoglycosides, is significantly reduced in acidic environments which has particular relevance for CF patients to whom these antibiotics are administered frequently.20
In conclusion, the pH of exhaled breath condensate in patients with stable CF is acidic and in an exacerbation is almost one log order lower than in healthy subjects. This appears to be related in part to inflammation as the pH of the exhaled breath condensate rises significantly during treatment of an exacerbation, although not to control values. It is possible that, even in stable disease, the CF airway cannot compensate for the background level of inflammation, leading to chronic acidification of the ELF. Given the deleterious effects this may have on the pathophysiology of the CF lung, further studies are required of pH regulation of the CF airway in vivo.
REFERENCES
Footnotes
-
Dr S Tate and the study were supported by MRC programme grant G9313618, Cystic Fibrosis Project LT3 and the UK Cystic Fibrosis Gene Therapy Consortium.