Article Text
Abstract
Recent studies have identified mucosal healing on endoscopy as a key prognostic parameter in the management of inflammatory bowel diseases (IBD), thus highlighting the role of endoscopy for monitoring of disease activity in IBD. In fact, mucosal healing has emerged as a key treatment goal in IBD that predicts sustained clinical remission and resection-free survival of patients. The structural basis of mucosal healing is an intact barrier function of the gut epithelium that prevents translocation of commensal bacteria into the mucosa and submucosa with subsequent immune cell activation. Thus, mucosal healing should be considered as an initial event in the suppression of inflammation of deeper layers of the bowel wall, rather than as a sign of complete healing of gut inflammation. In this systematic review, the clinical studies on mucosal healing are summarised and the effects of anti-inflammatory or immunosuppressive drugs such as 5-aminosalicylates, corticosteroids, azathioprine, ciclosporin and anti-TNF antibodies (adalimumab, certolizumab pegol, infliximab) on mucosal healing are discussed. Finally, the implications of mucosal healing for subsequent clinical management in patients with IBD are highlighted.
- Mucosal healing
- IBD
- endoscopy
- epithelial cells
- immunosuppression
- inflammatory bowel disease
- crohns disease
- infliximab
- 5-aminosalicylic acid (5-ASA)
- clinical trials
Statistics from Altmetric.com
- Mucosal healing
- IBD
- endoscopy
- epithelial cells
- immunosuppression
- inflammatory bowel disease
- crohns disease
- infliximab
- 5-aminosalicylic acid (5-ASA)
- clinical trials
Introduction
Does mucosal healing matter in inflammatory bowel disease (IBD)? The subject is receiving increasing attention1–6 as a goal of therapy beyond symptom control, as a primary endpoint in recent clinical trials and as a discriminant between classes of drugs. The answer is that it can only matter for patients if it serves as a surrogate marker for more effective disease control, meaning symptom and steroid-free remission that predicts a better course of disease. This in turn implies patient-related outcome measures, such as lower rates of hospitalisation or surgery and improved ability to work and live normally, with an improved quality of life. Mucosal healing as an appropriate goal for managing IBD is almost there, but suffers the downside of being defined by endoscopy, which in turn affects patients. Consequently surrogate markers of the surrogate marker of mucosal healing are being explored and clinicians are rightly circumspect about the implications.
Approach to systematic analysis
A Web of Knowledge search using the search term ‘mucosal healing IBD’ identified more than 10 manuscripts published per year since 2009 (2009: n=14; 2010: n=15; 2011: n=18) and more than 100 citations per year since 2008 (citations 2008: 119; 2009: 160; 2010: 243; 2011: 329). To structure and compare these observations, this systematic review on mucosal healing in IBD defines the term, evaluates the structural basis and then summarises the clinical studies on mucosal healing in IBD. Particular aims are to analyse the effects of anti-inflammatory or immunosuppressive agents on mucosal healing in IBD and to highlight potential implications of mucosal healing for the clinical management of patients.
Methods for generating inclusion criteria and analysis of data were based on PRISMA recommendations.7 ,8 A literature search using Medline and Science Citation Index in duplicate was performed in March 2012 and May 2012. All studies were reviewed that were published from 1992 to March 2012 in which mucosal healing in IBD was studied. Potential exclusion criteria to reduce risk of bias and unnecessary observations included case reports on single patients and book chapters. Relevant publications were identified for the period 1992–2012. The medical terms ‘mucosal healing (TI)’, ‘mucosal healing IBD’, ‘mucosal healing infliximab’, ‘mucosal healing adalimumab’, ‘mucosal healing certolizumab’, ‘mucosal healing azathioprine’, ‘mucosal healing methotrexate’, ‘mucosal healing ciclosporin’, ‘mucosal healing aminosalicylates’, ‘mucosal healing corticosteroids’ and ‘mucosal healing colitis’ were used in the search. Additional references in review articles were hand-searched. The full papers of all relevant studies were retrieved, and reference lists from identified papers were searched to identify any additional studies that may have been missed during the process. Potential studies were initially screened by title and abstract. A total of 251 articles were studied to construct this review. To reduce risk of bias, special attention was paid to the method used for the assessment of mucosal healing in all clinical studies. No funding for writing this review was obtained.
The meaning of mucosal healing: structural and functional components
Initial manuscripts using the term mucosal healing were published in the 1980s. These included studies on the effects of dentures on mucosal healing and bone remodelling,9 ischaemic necrosis of pony jejunum10 and the effects of laser or cryosurgery on the inflammatory reaction and tissue healing in sheep's tongues.11 Subsequent studies revealed that mucosal healing is a complex process involving epithelial cell migration and proliferation, regulated by a variety of autocrine and juxtacrine growth factors as well as conventional gut peptides.12 ,13 It is specifically regulated by soluble (circulating) factors, with phases that might be targeted by pharmacological agents.14 For instance, endogenous prostaglandins promoted mucosal healing in experimental colitis, as did leukotriene biosynthesis inhibitors, by shifting arachidonic acid metabolism towards production of prostaglandins, at least in murine models.15 ,16
Repeated intestinal epithelial damage with disruption of the intestinal barrier function is a key feature of IBD.17–19 Such alterations of intestinal barrier function are thought to result in translocation of commensal bacteria in the bowel wall, inducing uncontrolled T cell activation and chronic intestinal inflammation.20–23 Structural and functional alterations of the barrier in IBD affect the intestinal epithelial cells (IECs) themselves, tight and adherens junctions, desmosomes, mucins and defensins, which act as antimicrobial peptides.18 ,19 ,24 Tight junctions are semi-permeable gates regulating the passive movement of luminal fluid and passive diffusion of proteins and lipids, while adherens junctions regulate intercellular adhesion. Major macromolecular components in this apical junctional complex include occludin, claudins, junctional adhesion molecules, tricellulin and E-cadherin as transmembrane proteins.25 ,26 The expression of these proteins is tightly controlled by intracellular signalling and regulatory proteins. For instance, the mucosal protein AMP-18 appears to regulate expression of junctional adhesion molecules that facilitate assembly of multiple proteins into tight junctions, thereby improving mucosal barrier function.27 These details matter, because they are the potential molecular targets for therapy which give mucosal healing a functional meaning. Current methods assess healing only by white light endoscopy and do nothing to assess function, while the microscopic (let alone the ultrastructural) components are ignored.
Mechanisms of mucosal restitution and repair
Under normal circumstances, IECs are constantly shed from the tips of villi after cell death.28 ,29 To maintain intestinal homeostasis, controlled proliferation of IECs is required to prevent loss of epithelial barrier function. Stimuli that induce proliferation consist of ‘growth’ factors and soluble proteins that induce IEC activation. On binding to their ‘cognate’ receptor, intracellular signalling events augment proliferation, differentiation and survival of IECs. Consider epithelial growth factor (EGF) that binds to its receptor (EGFR) on IECs, activating intracellular kinases and transcription factors. These proteins mediate IEC proliferation that is essential for gap closure and healing of the mucosa.
Mucosal healing in IBD is therefore a tightly controlled process associated with suppression of inflammation and improvement of intestinal barrier function.30 It is also dependent on the balance of migration, proliferation and functional differentiation of IECs adjacent to the injured area. This is controlled by regulatory growth factors, peptides and immunoregulatory cytokines31–33 (figure 1), as well as activation of signalling pathways including toll-like receptors (TLRs) that are involved in intestinal epithelial wound healing. Following injury and evolution of erosions in IBD, adjacent IECs rapidly migrate into the denuded area to restore barrier integrity (‘epithelial restitution’).33 ,34 Proliferation of IECs then increases the number of enterocytes to resurface the defect. Further differentiation of IECs is needed to restore mucosal barrier and epithelial function. These three phases are facilitated by regulatory proteins such as chemokines (eg, CXCR4 and CXCL12), defensins and various growth factors,35 including transforming growth factor (TGF)-α and TGF-β, epidermal growth factor, fibroblast growth factor (FGF), keratinocyte growth factor (KGF), insulin-like growth factor (IGF)-I and IGF-II, hepatocyte growth factor (HGF), the cytokines IL-1β, IL-2, IL-6 and IL-22, and trefoil peptides33 ,36–38 (figure 1). While many of these proteins promote epithelial cell restitution through enhanced production of bioactive TGF-β (a central regulator of epithelial restitution), other factors such as trefoil peptides and galectins 2 and 4 promote it in a TGF-β-independent manner.32 ,36 ,39–41
Molecular signalling pathways driving mucosal healing. Signalling events in Paneth cells, intestinal epithelial cells and goblet cells contribute to mucosal healing. In particular, growth factors, antimicrobial peptides, cytokines, chemokines, trefoil factors and mucins play an important role in mucosal healing. Several of the depicted signalling pathways have been successfully explored for augmented mucosal healing in experimental models (eg, trefoil peptides; see text for details).
Specific signalling pathways play a crucial role in epithelial cell restitution. For instance, activation of the extracellular signal-regulated kinases ERK1/ERK2 and the mitogen-activated protein kinase control restitution after TGF-α signal transduction.42 Furthermore, Trem2 signalling promotes mucosal healing by regulating cytokine production (IL-4, IL-13).43 Furthermore, activation of the nuclear factor (NF)-κB pathway induces proliferation of IECs during mucosal injury.44 Finally, studies in conditional knockout mice indicate a crucial role of signal transducer and activator of transcription (STAT) 5 in driving cytokine-mediated wound healing.45 These data suggest that specific signalling mechanisms play a crucial role in mucosal healing at an epithelial level and all are targets for practice.
Murine models suggest that targeted modulation of signalling pathways can regulate mucosal healing in experimental colitis. For instance, genetic targeting of Smad5, a protein partly responsible for mediating Bmp signals in IECs, led to the dysregulation of IEC migration, increasing susceptibility to colitis and impairment in wound healing.46 Furthermore, activation of protein kinase C (PKC) signalling resulted in protection from TNF-induced tight junction disruption and mucosal healing in experimental colitis,47 suggesting that induction of PKC activity may improve mucosal healing. The p53-upregulated modulator of apoptosis PUMA also contributes to the pathogenesis of colitis by promoting IEC apoptosis48 and PUMA expression is markedly increased in ulcerative colitis (UC), correlating with IEC apoptosis, so PUMA inhibition may promote mucosal healing in patients with UC.
Innate immunity controls epithelial restitution. The innate immune-recognition TLRs bind to specific pathogen-associated molecular patterns of bacteria and viruses through nucleotide-binding oligomerisation domain-containing proteins.49 The gut flora is recognised by TLR2 and TLR4 under normal conditions, controlling IEC homeostasis.50 Activation by TLR2 or TLR4 ligands protects against mucosal injury and colitis, suggesting that TLR signalling is a target for modulating colitis, potentially by regulating trefoil factor 3 expression and preventing IEC apoptosis.51 ,52 The TLR5 agonist flagellin potentially protects IECs from bacteria-mediated apoptotic challenge through the activation of NF-κB and PI3K/Akt signalling.53 Nevertheless, a novel antibody against TLR4 ameliorated inflammation but impaired mucosal healing in experimental colitis,54 so TLR signalling has pleiotropic functions.
Signalling via TLR9, induced by bacterial DNA,55 contributes to intestinal homeostasis in IECs.56 ,57 TLR9 recognises unmethylated C plus G (CpG) containing sequences in DNA molecules that are frequently present in bacterial genomes. Consequently, bacterial DNA and CpG motifs can stimulate innate immune cells to produce immunoregulatory cytokines such as IL-12 or interferons. For instance, TLR9 agonists suppressed the severity of experimental colitis by inducing type I interferons (IFN-α/β). These findings suggest that apical exposure of IECs to luminal microbial DNA may support intestinal homeostasis via the activation of TLR9. Modulation of mucosal healing via bacterial products or live microorganisms (probiotics) containing CpG DNA thus emerges as a novel approach for colitis therapy.
So mucosal healing emerges as a finely-tuned and highly structured process. During healing, IECs receive numerous environmental signals, through growth factors (TGFα/β, EGF), cytokines (IL-6, IL-22) and/or bacterial products (LPS, CpG DNA). These factors induce specific intracellular signalling cascades leading to activation of master transcription factors such as NF-κB and STAT-3 in IECs. On activation, these key regulators mediate antiapoptotic and proliferative effects, leading to augmented survival and cell division in IECs. This hierarchical system favours healing of intestinal lesions and is essential for efficient wound healing in mucosal inflammation.
Several recent studies have addressed the structural consequences of mucosal healing in IBD. Ultrastructural changes in mucosal healing can be identified within 4 weeks of anti-TNF antibody therapy for UC.58 Treatment reversed or improved microvillous depletion, shattering of the epithelial junctions, cytoplasmic vacuolisation, pyknotic nuclei, structural changes in mitochondria and Golgi complexes, abnormal mucus formation and infiltrating mononuclear cells.58 Anti-TNF therapy has also been reported to induce regulatory macrophages, which promote wound healing in an in vitro model.59 ,60 Furthermore, adalimumab, certolizumab pegol and infliximab all appear to suppress inflammation and promote mucosal healing by blockade of TNFR2-mediated T cell resistance against apoptosis.61 The clinical consequences of ultrastructural healing in addition to histological and endoscopic mucosal healing remain to be determined, but they imply molecular mechanisms open to manipulation.
Clinical definition of mucosal healing in IBD
In the context of IBD, the term mucosal healing refers to endoscopic assessment of disease activity and is usually taken to reflect resolution of visible ulcers. The advantage of ‘absence of ulcers’ is that it is readily recognised in Crohn's disease,3 but it represents a target that is difficult to achieve. For instance, just 15/62 patients achieved this goal on induction and maintenance adalimumab at 12 weeks and 15/62 after 1 year in the EXTEND study, which was the first clinical trial of biological therapy to use mucosal healing as a primary endpoint.3 Furthermore, absence of ulcers is a binomial endpoint that does not quantify grades of improvement, so endoscopic scoring systems are deployed without much agreement on clinically relevant thresholds. In Crohn's disease, the most frequently used scores comprise the Crohn's disease endoscopic index of severity (CDEIS), the simplified index SES-CD (simple endoscopic score for Crohn's disease, where SES-CD=0 equates to absence of ulcers), or the Rutgeerts score (to evaluate the anastomosis after ileocolic resection)62–66 (table 1). While the former two scores have been validated, the latter has not. The CDEIS is a gold standard for the assessment of endoscopic activity of Crohn's disease but is complex, subject to interobserver variation and concentrates on the presence of ulcers, with a range from 0 to 44 points. For instance, owing to inter-observer variation, central reading of video endoscopies was necessary after trial completion in an otherwise well conducted open label study of certolizumab.69 The threshold for endoscopic remission has been set as a CDEIS <6, with other criteria for response (a decrease in CDEIS >5), complete endoscopic remission (CDEIS <3) and mucosal healing (absence of ulcers). The implications, highlighted in figure 2, are that a patient with active inflammation due to Crohn's disease in the last 20 cm of the terminal ileum will have a score of 4 in the absence of ulcers or stenoses elsewhere, and this would be classified as ‘endoscopic remission’. Furthermore, the complexity of the CDEIS does not lend itself to use in daily routine. On the other hand, the persistence of some endoscopic lesions in Crohn's disease does not appear to influence the outcome over the course of a year. In the MUSIC study of certolizumab pegol in Crohn's disease, maintenance of improvement between weeks 10 and 54, based on individual patient data, was found in about 70% of those who responded (decline in CDEIS >5) and those with complete remission (CDEIS <3), and in over 40% of those with remission (CDEIS <6). In addition, the CDEIS showed no significant change in the ITT population from week 10 to week 54, although it did increase slightly in those with an endoscopic evaluation at both time points.69 The difficulty in defining thresholds is first that the severity of endoscopic lesions at the start affects the clinical implications of the endpoint (response, remission or absence of ulcers). Second, a clinically relevant duration of follow-up has yet to be agreed, although one that can also be shown to alter the rate of surgery or hospitalisation seems appropriate.
Endoscopic disease activity indices for Crohn's disease
The CDEIS score for assessment of mucosal healing in Crohn's disease. One example for a patient with moderate ileal inflammation in the absence of ulcers and stenosis is given.
Mucosal healing is paradoxically more difficult to define in UC, since it is uncommonly associated with visible ulceration. There are many scoring systems (Baron and modified Baron score, the Mayo Clinic score, Sutherland, Powell-Tuck and Rachmilewitz indices, among others)70–78 (table 2), although none have been validated. The Mayo Clinic endoscopy subscore has been most commonly deployed, defining mucosal healing as a score of <1 (normal mucosa or loss of vascular pattern, but no mucosal friability), when the endoscopy subscore was 2 or 3 at trial entry.79–82 Interestingly, a Mayo Clinic endoscopy subscore of 0/1 in infliximab-treated patients predicted a better outcome as far as avoiding colectomy was concerned, than did a score of 2/3.79 However, significant differences between subscores 0 and 1 at week 8 were observed with regard to prediction of corticosteroid-free status (week 30: n=40/65 vs 47/102; 62% vs 46%; week 54: n=20/32 vs 25/54; 63% vs 46%; p<0.0001) and corticosteroid-free symptomatic remission (week 30: n=30/65 vs 35/102; 46% vs 34%; week 54: n=15/32 vs 19/54; 47% vs 35%; p<0.0001) at weeks 30 and 54. This is in spite of the fact that inter-observer variation in endoscopic assessment of UC is so large that there was only 27% concordance in the classification of remission by experienced investigators.78 Consequently, the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) was developed as the first validated index of endoscopic severity. The final UCEIS model incorporates three components (vascular pattern, bleeding, erosions and ulcers), each with precise definitions and three or four levels of severity, yielding a 9-point scale that accounts for 94% of the variance between observers in the overall assessment of severity. It appears that the UCEIS allows precise overall assessment of endoscopic severity of UC, although the sensitivity of the index to change and a threshold for mucosal healing have yet to be determined.
Endoscopic disease activity indices for ulcerative colitis (UC)
Recent developments in endoscopy for assessment of IBD include more sophisticated techniques such as high definition endoscopy, magnification endoscopy, filter endoscopy and chromoendoscopy for cancer detection (figures 3 and 4).83–86 In addition, new techniques for in vivo histology have been developed. In particular, confocal laser endomicroscopy has emerged as a valuable tool for gastrointestinal endoscopic imaging in IBD.87 ,88 Endomicroscopy enables the endoscopist to obtain real time in vivo histology during endoscopy for the generation of optical biopsies. Recent studies have used endomicroscopy for in vivo assessment of endoscopic activity in IBD. In a study in 54 patients with Crohn's disease, Neumann et al 67 developed an endomicroscopic activity index, denoted Crohn's Disease Endomicroscopic Activity Score, to determine the severity of inflammation in this disease. Although this score needs validation, it represents the first endoscopic index for Crohn's disease based on in vivo histology (figure 3). This has yet to be applied to UC. Furthermore, Kiesslich et al 68 recently developed a scoring system (Watson score) to assess local barrier dysfunction in Crohn's disease in vivo that was used in a study with 51 patients. This is based on functional in vivo imaging that may be used to predict clinical relapses, although validation studies are required. Nevertheless, novel imaging technologies will undoubtedly provide unique insights into the structure and function of the bowel wall that will lead to innovative predictors of clinical relapse in Crohn's disease.
Analysis of the mucosa by using fluorescein-aided confocal laser endomicroscopy in inflammatory bowel disease (IBD) (B–D) and control (panel A) patients. Normal mucosa is seen in (A), while (C) illustrates signs of active Crohn's disease with decreased numbers of colonic crypts, dense cellular infiltrate within the lamina propria and fluorescein leakage due to extravasation of fluorescein sodium. In (B), endomicroscopic findings in clinically inactive Crohn's disease (CDAI <150 points, CDEIS <4) are shown. In spite of lacking signs of endoscopically active disease, endomicroscopy highlights an increased number of colonic crypts and augmented crypt tortuosity and branching of dilated crypts. (D) illustrates an example of endomicroscopic mucosal healing in Crohn's disease with normal crypt structure.
High definition endoscopic images of patients with active Crohn's disease (CD) and ulcerative colitis (UC) are shown. In addition, examples of endoscopic mucosal healing (MH) are given for both diseases. Drugs that have been described to promote mucosal healing in CD and UC are highlighted. The levels of evidence (USPSTF) for the induction of MH are highlighted by different colours.
Clinical relevance of mucosal healing in Crohn's disease
The clinical relevance of mucosal healing in patients with IBD was first highlighted when treatment with azathioprine was shown to promote mucosal healing in Crohn's disease.89 ,90 Azathioprine is an immunosuppressive agent that suppresses the activity on the small GTPase Rac1 in T cells, thereby inducing programmed cell death (apoptosis) of activated intestinal lymphocytes in IBD.91 ,92 In the mid-1990s D'Haens et al 89 studied 19 patients with recurrent ileitis and noted on endoscopy that azathioprine therapy in the absence of corticosteroids led to complete macroscopic healing (absence of ulcers) in 40%, near complete healing in 33% and partial healing of the mucosa in 20% of patients after 6 months. The clinical value of this observation was unclear at that time, because there was little correlation between endoscopic mucosal healing and clinical remission of disease, as determined by the Crohn's disease activity index (CDAI).93 Furthermore, endoscopic evidence of mucosal healing was not necessarily associated with histological evidence of suppression of inflammation. In a study on 38 patients with Crohn's disease, histological evidence of activity persisted in one-third of patients in whom endoscopic evidence of mucosal healing was present.94 Furthermore, there was no clear correlation between mucosal healing and relapse rates in corticosteroid-treated patients, so a symptom-oriented approach to Crohn's disease was customary in the 1990s. This view was challenged by studies using biological (anti-TNF antibody) therapy, in which the induction of mucosal healing was very notable.95 In the initial open-label study, 8/10 patients showed normalisation of CDAI scores and healing of ulcers as judged by endoscopy within 4 weeks of treatment. Large controlled studies provided unequivocal evidence that infliximab can both induce and maintain remission in a significantly higher percentage of patients with Crohn's disease compared to placebo.96
A pivotal question was whether severe ulceration in Crohn's disease might predict a higher risk of colectomy or penetrating complications. Allez et al 97 studied 102 patients with Crohn's disease, of whom 53 had extensive and deep ulceration at index colonoscopy, covering more than 10% of the mucosal area of at least one segment of the colon. The risk of colectomy was independently affected by the presence of ulceration at index colonoscopy. The probability of colectomy was 31% and 6% at 1 year, 42% and 8% at 3 years, and 62% and 18% at 8 years in patients with and without extensive and deep ulcerations, respectively. This showed that patients with deep and extensive ulcerations due to Crohn's disease have a more aggressive clinical course, with an increased rate of penetrating complications and surgery. If deep ulceration predicts an aggressive course of Crohn's disease, then mucosal healing might predict a favourable course of the disease with a reduced risk of complications. In a study of 183 patients, Schnitzler et al 2 found that mucosal healing during maintenance infliximab treatment was associated with an improved long-term outcome and a lower rate of major abdominal surgery in particular. Furthermore, in the ACCENT I endoscopic substudy,98 no patients with mucosal healing at weeks 10 or 54 needed hospitalisation, compared to 4/16 (25%) patients showing mucosal healing at only one of these time points and 34/74 (46%) with no mucosal healing, suggesting that mucosal healing predicts a reduction in hospitalisation.
A further question was whether mucosal healing really has long-term effects in a condition that currently lasts a lifetime. A population-based cohort study evaluated the association between mucosal healing 1 year after diagnosis and disease outcome over the next 7 years in 227 patients with Crohn's disease.99 Mucosal healing was associated with less inflammation (p=0.02), a decreased need for steroid treatment (p=0.02) and a trend to a lower resection rate over the next 7 years, with 6/50 patients showing mucosal healing at 1 year undergoing resection, compared to 18/80 patients showing endoscopic activity (p=0.1). Perhaps most impressively, however, the long term follow-up of 49/133 patients in the ‘step up/top down’ study70 showed that those patients who achieved complete mucosal healing (absence of ulcers, SES-CD=0) at 2 years had a higher rate of clinical remission (p=0.036), steroid-free remission (p=0.01), and steroid-free remission without flare through years 3 and 4.100
Effects of drugs and enteral nutrition on mucosal healing in Crohn's disease
Almost all studies in Crohn's disease use the CDAI as the primary endpoint, although many assess mucosal healing as a secondary endpoint.96 The notable exception is the EXTEND study of 135 patients with Crohn's disease,101 which used complete mucosal healing (absence of ulcers) as the primary endpoint.
Corticosteroids
A study in 1990 on the effects of prednisolone on mucosal healing in patients with active Crohn's disease found that 27% of patients had minor lesions and 12% complete mucosal healing after 4–7 weeks of therapy.65 Similarly, 0/8 patients with Crohn's disease treated with prednisolone for postoperative recurrence showed mucosal healing after 6–9 weeks, based on the overall endoscopic assessment of the mucosa rather than a detailed endoscopic score.102 These findings suggest that corticosteroids have little or no positive effects on induction of mucosal healing in Crohn's disease. Interestingly in the IBSEN cohort of 227 patients, medical treatment without steroids was a predictor for mucosal healing in Crohn's disease,99 raising the possibility that corticosteroids may actually interfere with mucosal healing. In any event, corticosteroids appear incapable of maintaining clinical remission, let alone mucosal healing, in Crohn's disease.103 ,104
Azathioprine
In contrast to corticosteroids, azathioprine seems to promote mucosal healing in Crohn's disease. In 1995, an 1800-mg intravenous loading dose of azathioprine over 36 h followed by oral administration (50 mg/day) was safe in Crohn's disease. Out of 6/12 patients with active endoscopic inflammation, 3/6 showed mucosal healing at week 16 suggesting that azathioprine favours mucosal healing. Azathioprine therapy in the absence of corticosteroids led to complete or near complete endoscopic healing in 73% of 19 patients after 6 months,89 so the concept that azathioprine rather than corticosteroid treatment induces mucosal healing was tested. The SONIC trial used mucosal healing as a secondary endpoint to compare the efficacy of azathioprine and infliximab monotherapy with combination therapy in patients with Crohn's disease who had never received immunomodulators or biological therapy. Only 30% (51/170) of azathioprine-treated patients achieved corticosteroid-free clinical remission and just 16% (18/109) had mucosal healing at week 26,96 which is appreciably less than the open label experience. The effect of azathioprine may take months to appear and clinical efficacy was reported before mucosal healing in a study of 16 patients.105 This concept of delayed mucosal healing is consistent with the delayed mechanism of action of azathioprine for the induction of T cell apoptosis.91 Unlike corticosteroids, however, azathioprine is capable of maintaining mucosal healing. A prospective randomised study comparing azathioprine and budesonide for active Crohn's disease used mucosal healing as a primary endpoint104 long before trials with biological therapy. Seventy-seven patients in clinical remission after conventional steroids were randomised to azathioprine or budesonide treatment for 1 year. Despite similar scores at baseline, the CDEIS only fell significantly in the azathioprine group by the end of the study at 1 year. Complete or near-complete healing was achieved in 25/30 (83%) of azathioprine-treated patients compared with only 6/25 (24%) of budesonide-treated patients (p<0.0001). A similar effect of azathioprine on mucosal healing was found in another small study on 20 patients with Crohn's disease in clinical remission after azathioprine for at least 9 (mean 24.4±13.7) months and no corticosteroids for at least 3 months106: there was complete healing in the colon in 70%, near-complete healing in 10%, partial healing in 15%, and no healing in 5%, suggesting that clinically successful azathioprine therapy is accompanied by mucosal healing in most cases. In those with ileal disease, complete healing was detected in 54%, near-complete healing in 15%, partial healing in 8% and no healing in 15%, so there may be different susceptibilities to mucosal healing within the gastrointestinal tract, although this has only been examined in small numbers.
Methotrexate
When methotrexate (MTX) was given as a 25 mg intramuscular injection weekly for 12 weeks in a pilot study and then switched to a tapering oral dose if clinical improvement was noted,107 5/14 patients with Crohn's disease given MTX had mucosal healing after 12 weeks compared to 0/7 in a group with UC. Other small studies have consistently found that MTX promotes mucosal healing in some patients with Crohn's disease,108 ,109 but when compared to azathioprine or infliximab,110 mucosal healing was reported in 2/18 (11%) patients on MTX, 9/18 (50%) on azathioprine (p=0.011 vs MTX) and 9/15 (60%) on infliximab (p=0.008 vs MTX). Consequently, although MTX may promote mucosal healing, it may be less frequently achieved than on azathioprine or infliximab.
Biological therapy
Encouraged by the initial open label studies,95 many studies have now examined the effects of adalimumab, certolizumab pegol, infliximab and natalizumab on mucosal healing in Crohn's disease. The ACCENT I study in 2002111 analysed mucosal healing (absence of ulcers) in a subgroup of 99 patients at week 10. It was found that infliximab (5 mg/kg at weeks 0, 2 and 6) led to mucosal healing in 29%, compared to 3% patients who received only one infusion at baseline. The CDEIS declined from a median 6.5 at baseline to 2.1 at 10 weeks. Subsequent scheduled maintenance therapy with infliximab (every 8 weeks) led to mucosal healing in 44% of patients at week 54, compared to 18% given episodic treatment.112 Although not all patients underwent control colonoscopies in these studies, it clearly suggested that induction and maintenance therapy with infliximab favoured mucosal healing. The SONIC trial96 used a similar approach (5 mg/kg at weeks 0, 2 and 6 followed by maintenance therapy every 8 weeks) and found mucosal healing in 28/93 (30%) infliximab-treated patients at week 26, compared to 18/109 (17%) patients on azathioprine monotherapy (p=0.02 vs infliximab) and 47/107 (44%) patients on combination therapy with azathioprine plus infliximab (p=0.06 vs infliximab monotherapy). A more recent retrospective study in 71 patients with Crohn's disease showed mucosal healing at first follow-up endoscopy in 45% of infliximab-treated patients at 3 months, which was highly predictive for its persistence at 12 months, maintained in 90% of patients, when infliximab was continued. Endoscopy at 3 months might therefore be used to predict responders to infliximab for maintenance therapy in active luminal CD.113
With regard to adalimumab the EXTEND trial evaluated adalimumab 160/80 mg at weeks 0/2 and maintenance 40 mg every other week for induction and maintenance of mucosal healing in 135 adults with ileocolonic Crohn's disease.3 Twenty-seven per cent of patients receiving adalimumab reached the primary endpoint (mucosal healing) at week 12 vs 13% given placebo. At week 52, rates of mucosal healing were 24% and 0%, respectively. It is not surprising that following induction therapy with adalimumab, patients with moderately to severely active CD who continue to receive adalimumab are more likely to achieve mucosal healing than those given placebo, but what matters is that those who achieved complete mucosal healing also avoided hospitalisation. Even though the target of mucosal healing may be achieved in a minority of patients, this matters to those who do.
The effect of certolizumab pegol on mucosal healing in Crohn's disease (MUSIC) has been highlighted above,69 because it developed definitions for endoscopic response and remission. The primary endpoint was the mean change in CDEIS from baseline at week 10, which declined from 14.5 to 8.8 in the ITT population (mean decrease 5.7, 95% CI 4.6 to 6.8, p<0.0001) of 89 patients (only 53 of whom had endoscopic evaluation at baseline, week 10 and week 54). Among several notable features of this study were the endoscopic severity at baseline (ulceration in ≥2 intestinal segments and a CDEIS ≥8 points), comparisons between central and local reader (the central reader always scored lower) and correlation with histology (correlation coefficient 0.24 at week 10). At week 10 (and week 54) rates of endoscopic response (a decrease in CDEIS >5) were 54% (49%) and 37% (27%) for endoscopic remission (CDEIS <5), 10% (14%) for complete endoscopic remission (CDEIS <2) and 4% (8%) for complete mucosal healing (CDEIS=0). The change in the mean CDEIS score (the primary endpoint) was from 14.7 to 8.3 (−6.5, 95% CI −7.6 to −5.3). These findings suggest that certolizumab pegol favours mucosal healing at week 10 and can maintain mucosal healing through week 54. It is important to point out, however, that a direct comparison of these data with findings from the above controlled clinical studies on infliximab and adalimumab (ACCENT I, SONIC, EXTEND) is difficult because of differences in study design, selection of patients and definition of mucosal healing. It should be noted, however, that the main limitation of the MUSIC trial was the lack of a control group. Thus, prospective controlled studies on the effects of certolizumab pegol on mucosal healing in CD with comparable endpoints to previous anti-TNF trials are highly warranted.
The α4 integrin antagonist natalizumab is not available in Europe, but 53 patients were evaluated in a colonoscopy substudy in the ENACT-1 trial evaluating the efficacy of natalizumab for active CD.114 Of those with ulcerations at study entry and treated with natalizumab (n=38), 22% showed complete mucosal healing, compared to 8% in the placebo group (n=15), suggesting that natalizumab can induce mucosal healing.
Induction of mucosal healing is one thing, but maintenance (the durability or sustainability) of response is another. This is increasingly evaluated by the proportion of patients who achieve mucosal healing at both early (6, 8 or 10 weeks) and late (54 week) time points. Infliximab can maintain mucosal healing. All patients who achieved mucosal healing after infliximab treatment (n=9) at both weeks 10 and 54 did not require any hospitalisation, while patients with mucosal healing at only one of those visits still required fewer hospitalisations (19%, n=3/16) compared to those who did not have mucosal healing at either visit (28%, n=14/50).98 Taken together with the 3- and 4-year results of the ‘step up/top down’ study,70 complete mucosal healing in patients with Crohn's disease has a sustainable effect on steroid-free remission rates, surgery and hospitalisation.
Enteral nutrition
Paediatric studies have suggested that enteral nutrition may affect mucosal healing in Crohn's disease. Borelli et al 115 compared, in a small study, corticosteroid therapy with enteral nutrition, and found a significantly higher rate of mucosal healing on treatment with polymeric diet as compared to corticosteroid therapy (14/19 vs 6/18; 74% vs 33%; p<0.05). Case studies concur.116 ,117 Finally, a retrospective study suggested similar rates of mucosal healing in patients receiving fractionated or continuous enteral feeding.118 Taken together, there is limited evidence that enteral nutrition favours mucosal healing. However, large prospective studies in children and adult patients are missing.
Clinical relevance of mucosal healing in UC
In UC, mucosal healing has long been recognised as a therapeutic goal.6 ,119–121 However, some studies have noted that endoscopic and microscopic changes in the rectum can persist despite apparent resolution of symptoms.122 ,123 This was first demonstrated by Truelove and Richards in 1956124 and has been confirmed by others.125 ,126 It also became clear from these studies that histological changes are often present despite endoscopic mucosal healing. In 1991, Riley et al determined whether such changes influenced clinical outcomes.127 They studied patients in clinical remission and found that those with histological features of acute inflammation on rectal biopsy had a higher incidence of relapse in the subsequent year. A Canadian group subsequently studied 74 patients with inactive UC and noted that basal plasmacytosis on rectal biopsy specimens was a significant predictor (p=0.003) of shorter time to clinical relapse.128 It should, however, be noted that clinical remission of disease activity on corticosteroid therapy in UC is often not accompanied by endoscopic mucosal healing.126
As far as the long term implications of mucosal healing in UC are concerned, the IBSEN cohort of 513 patients found that mucosal healing 1 year after diagnosis was significantly (p<0.05) associated with a lower risk of colectomy over the next 5 years.99 Although only 8% of patients required colectomy within 5 years in the absence of mucosal healing at 1 year, just 2% needed colectomy when there was mucosal healing. This is consistent with a post-hoc analysis of the large ACT1/2 trials79 which showed that infliximab-treated patients with an endoscopy subscore of 0 or 1 at week 8 had a significantly lower risk of colectomy over the next year, compared to patients with scores of 3 or 4 (p=0.0004), as well as better symptom control and a lower rate of steroid use. Interestingly (in spite of inter-observer variation), a Mayo Clinic endoscopy subscore of 0 discriminated a score of 1 with regard to symptoms and steroid-dependency, although not colectomy: an endoscopy subscore of 0 at week 8 predicted symptom relief (stool frequency or 1–2 more than normal each day, but no rectal bleeding) at weeks 30 and 54 in 71% and 74%, respectively, compared to 51% and 47% for a score of 1 at week 8.79 Patients with an endoscopic subscore of 0 at week 8 in the ACT 1 trial had a higher rate of steroid-free remission at week 54 (63%, 22/32) than those with a score of 1 (46%, 25/54). These findings suggest that mucosal healing in UC predicts the subsequent course of disease, as it does in Crohn's disease.
An important concern is the potential relationship between mucosal healing and cancer in UC. Previously severe inflammation (as indicated by post-inflammatory polyps) and persistent inflammation were independently associated with the risk of colorectal neoplasia during surveillance in the St Mark's cohort,129 suggesting that anti-inflammatory therapy might reduce the risk of neoplasia. Although controlled prospective studies are lacking, a large cohort study from Italy indicates a lower risk of colorectal cancer in patients receiving azathioprine therapy for up to 17 years in the presence of mucosal healing.130 Data from the CESAME cohort also reported a threefold decrease in the incidence of colorectal cancer in patients with extensive colitis for more than 10 years in those receiving azathioprine.131 Although other studies have not observed an azathioprine-associated reduction in the risk for colon cancer in UC, this suggests that the immunomodulatory properties of thiopurines may have a clinically meaningful effect on cancer development in colitis.
Effects of drugs on mucosal healing in UC
5-Aminosalicylates
Some studies have shown that 5-aminosalicylates (5-ASA) favour mucosal healing in UC.132–134 A placebo-controlled trial compared rates of mucosal healing in patients with mild to moderately active UC receiving MMX mesalazine.135 Although 65/85 (78%) patients receiving 4.8 g MMX mesalazine showed mucosal healing at week 8, mucosal healing was also detected in 40/86 (47%) patients on placebo. Significant differences were also found in another study, with mucosal healing in 32% in the MMX mesalazine group (2.4 g/day: 55/172; 4.8 g/day: 56/174) compared to 16% (27/171) on placebo.136 ,137 A third study found that mesalazine (3 g/d vs 1 g/three times daily) resulted in mucosal healing in 71% (135/191) and 70% (132/189) patients at week 8.134 Finally, the randomised clinical trials ASCENDI/II of delayed-release oral mesalazine 4.8 g/day vs 2.4 g/day showed mucosal healing (endoscopy subscore of 0 or 1) in 80% of patients with moderately active UC given 4.8 g/day and 68% on 2.4 g/day for 6 weeks (p=0.012).138 This suggests that higher doses of 5-ASA may provide additional benefit for mucosal healing in UC. The marked variability in mucosal healing rates in the studies on UC can be attributed to inter-observer variation in endoscopy, different time points and different scoring systems (sigmoidoscopic score of 0 or 0–1 vs Rachmilewitz index <4). Nevertheless, these findings demonstrate that mesalazine promotes mucosal healing in UC.
Corticosteroids
In contrast to Crohn's disease, corticosteroids appear able to induce mucosal healing in UC.139–148 As early as the mid-1950s, 120 patients with UC given high doses of oral corticosteroids (100 mg/day) had higher rates of mucosal healing compared to placebo (30% vs 10%) within 6 weeks.72 ,139 Mucosal healing in a study of 49 patients with acute severe colitis has also been reported after a 5-day intensive intravenous corticosteroid regimen.140 Studies with oral budesonide and corticosteroid enemas have detected mucosal healing in UC, suggesting that corticosteroids favour mucosal healing independent of the route of administration. For instance, a double-blinded study in active left-sided UC showed no differences between mucosal healing in patients receiving 10 mg budesonide daily (n=34) and patients given 40 mg prednisolone orally daily (n=38; mean sigmoidoscopic score at week 9: 1.5 vs 1.4).149 The paradox is that severe endoscopic ulceration predicts failure to respond to corticosteroids: in 85 consecutive patients,150 severe endoscopic lesions were associated with an increased risk of intravenous corticosteroid failure (p=0.007). The difficulty is in defining severe endoscopic lesions.
Azathioprine
An early placebo-controlled study examined mucosal healing in 80 patients with UC receiving corticosteroids plus azathioprine (2.5 mg/kg/day) or corticosteroids plus placebo.151 Numerically higher rates of mucosal healing (sigmoidoscopic score 0/1) with azathioprine (35/38, 92%), compared to 27/38 (71%) on placebo after just 4 weeks of therapy suggest that azathioprine favours mucosal healing in UC. However, this difference was not significant, but it should be noted that a relatively small number of patients was studied only. An open-label study with azathioprine for UC showed mucosal healing in 22/32 (69%) patients after at least 6 months' therapy, indicating that mucosal healing may be maintained with azathioprine.108 Another Italian study found that azathioprine therapy for 6 months in UC resulted in mucosal healing and complete remission in 19/36 (53%) patients compared to 7/36 (19%) patients on 5-ASA.152 These data probably overestimate the effect of azathioprine, because the prospective study comparing azathioprine monotherapy with infliximab or the combination of the two in 231 patients over 4 months found healing in 37%, 55% and 63%, respectively.153 Taken together, these data suggest that azathioprine induces mucosal healing in up to half of patients treated for at least 6 months,154 while combination therapy of azathioprine with corticosteroids or infliximab may lead to more rapid induction of mucosal healing compared to monotherapy. The potential role of azathioprine in reducing colitis-associated cancer119 presumably reflects better control of inflammation and mucosal healing.
Calcineurin inhibitors
Although the Leuven group did not find a significant effect on mucosal healing after ciclosporin (CsA) by day 8,155 CsA was more effective than steroids at inducing mucosal healing by week 4.156 More recently, a retrospective analysis of 72 patients with severe UC treated with CsA found that endoscopic improvement at day 14 was associated with a lower risk of colectomy at 1 year.157 Overall, 53/72 (74%) patients responded initially to CsA and 54% of patients required a colectomy within 11 years. The practical clinical message is that early mucosal healing helps to predict subsequent colectomy over the next year. The same is likely to apply to tacrolimus. In a double-blind multicentre study in steroid-refractory UC, 62 patients received oral tacrolimus or placebo for 2 weeks.158 Mucosal healing (endoscopy subscore 0 or 1) was 14/32 (44%) in the tacrolimus group and 4/30 (13%) in the placebo group (p=0.012), despite an even lower clinical remission rate which did not reach significance (p=0.238). This suggests that tacrolimus can induce rapid mucosal healing in UC, although there is a disparity with the short term clinical benefit.
Biological therapy
The ACT1/2 trials79 showed that infliximab induces high rates of mucosal healing (ACT1: 75/121, 62%; ACT2: 73/121, 60%) compared to placebo (ACT1: 41/121, 34%; ACT2: 38/123, 31%). The high rates of mucosal healing in the placebo group illustrate the problems with assessment and endoscopic scoring in UC. Scheduled maintenance therapy with infliximab every 8 weeks in ACT1 was associated with mucosal healing in 46% of patients at week 54, compared to 18% in the placebo group (p<0.001), suggesting that infliximab can maintain mucosal healing in a proportion with treatment-refractory UC. This is similar to a small study in just 17 patients with UC treated with infliximab, which maintained mucosal healing in patients with steroid-dependency.159 The effect of infliximab in UC may be durable. In the 3-year follow-up of the randomised trial of infliximab for UC failing intravenous steroids,160 none of the patients in endoscopic remission at 3 months after infliximab induction (0/8) had a colectomy, compared to 7/14 patients who were not in endoscopic remission (p=0.02).
Adalimumab also achieves mucosal healing. In a combined analysis of induction and maintenance studies, mucosal healing occurred in 43% on adalimumab (n=470) and 33% in the placebo group (n=468, p=0.002) at 8 weeks.101 ,161 There are, however, still some discrepancies. The ULTRA1 study in 390 patients showed no difference in mucosal healing between placebo (42%) and adalimumab groups (80 mg/40 mg: 38%; 160 mg/80 mg: 47%), but the ULTRA2 study in 494 patients reported significant (p=0.032) differences between placebo (31%) and adalimumab (160/80 mg then 40 mg every other week: 41%).101 ,161 The discrepancies might be due to the higher placebo response rate in ULTRA1 compared to ULTRA2. Furthermore, case series suggest that adalimumab favours mucosal healing.162 ,163 Finally, recent findings suggest that the anti-TNF antibody golimumab induces mucosal healing.80 In fact, treatment with golimumab at weeks 0 and 2 significantly induced mucosal healing at week 6 as compared to placebo therapy (400 mg/200 mg: 45%; p=0.0001; 200 mg/100 mg: 43%; p=0.0005 vs placebo: 29%) suggesting that several anti-TNF antibodies favour mucosal healing in UC.
As with Crohn's disease, durability of response matters in UC. Sustained mucosal healing (at weeks 8 and 52) was a prespecified endpoint in the combined analysis of ULTRA1 and ULTRA2 and was significant. Most recently, vedolizumab (an oral α4β7 integrin antagonist) for UC (300 mg intravenous at days 1 and 15, n=225 vs placebo, n=149) achieved mucosal healing (Mayo score <2) at week 6 in a significantly higher percentage of patients as compared to placebo (41% compared to 25% on placebo),81 suggesting that blockade of T cell homing in the gut may favour mucosal healing in UC.
Practice points
Mucosal healing in both UC and Crohn's disease can be achieved by several different drugs and is associated in both conditions with a better clinical outcome (steroid-free remission, lower rate of hospitalisation and surgery).164 ,165 Mucosal healing has therefore become an important endpoint in clinical trials3 and treatment paradigms have evolved towards a rapid escalation of therapy to achieve more stringent goals than improvement of clinical symptoms, including mucosal healing.166
The question is what to do in clinical practice, because patients understandably dislike invasive endoscopic procedures. It is nevertheless likely that endoscopy will be increasingly used to guide therapeutic decision-making,6 especially before starting, altering the dose, switching or stopping expensive biological therapy. The potential value of endoscopically-guided therapy is shown by observations in 230 children with IBD,167 showing that those with mucosal injury were very much more likely to have a management change than those with mucosal healing (80% vs 20%; p<0.001). Endoscopy is already commonly performed within a year of ileocolic resection and recommended by some guidelines to guide prophylactic therapy,168 although the results of an interventional study are awaited. Many clinicians with a special interest in IBD would already escalate treatment if the postoperative Rutgeerts' score was >i2 within 6–12 months of an ileocolic resection, be that initiating thiopurine therapy, dose-optimising through metabolite monitoring, or (re-)introducing anti-TNF therapy as appropriate.
The other occasion that already merits endoscopically guided decision-making is in stopping anti-TNF therapy. In a prospective study of stopping infliximab in 115 patients with Crohn's disease169 when in corticosteroid-free remission after treatment for at least 1 year with scheduled infliximab and an antimetabolite, multivariate analysis showed that endoscopic mucosal healing (CDEIS=0) identified a subgroup of patients which, when combined with a low CRP, normal haemoglobin and clinical history, could predict sustained remission in about 80%. Appropriate steps to take when considering whether to continue anti-TNF therapy (driven at times by the patient and at others by remuneration bodies), start with the context of the clinical decision, meaning the impact that a relapse will have on the patient. Were a relapse to jeopardise a university degree or other major life event, then it is difficult to justify stopping, whatever the endoscopy shows. The second consideration is the perception of the patient and only the third is the procedure, looking for complete mucosal healing (absence of ulcers), which will provide better support for a decision than clinical judgement alone. Only then (once antimetabolite therapy is re-established and contingency plans made in case of relapse) should anti-TNF therapy be withdrawn.
Future perspective
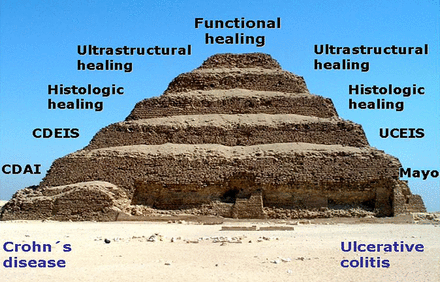
Modern endoscopic techniques such as spectral imaging, filter endoscopy, dye-based endoscopy, magnifying endoscopy, double balloon endoscopy, capsule endoscopy and endomicroscopy,86 ,170–172 for assessing mucosal healing are likely to assist decision-making in the future. It is possible that microscopic imaging of mucosa173 or assessment of barrier function68 may be necessary to evaluate functional as well as structural healing of chronic inflammation in IBD, leading to speculation that we will see a stepwise evolution of endpoints in clinical trials, towards more ambitious endpoints such as histological healing, ultrastructural healing and even functional healing of the mucosal barrier (figure 5). Indeed, initial studies suggest that histological healing or endomicroscopic healing may be used for optimised assessment of mucosal healing in IBD,67 ,68 ,127 although prospective studies with established endoscopic scoring systems are lacking. In any case, an intensive search for surrogate markers of mucosal healing is afoot.
Hypothetical stepwise evolution of endpoints in clinical inflammatory bowel disease trials towards functional barrier healing of the mucosa. While most studies mainly used primary clinical endpoints such as CDAI reduction, several recent studies have used endoscopic mucosal healing as primary endpoint. More ambitious endpoints such as histological or ultrastructural healing or even functional healing may be employed in future studies.
To avoid invasive endoscopy, alternative methods for the assessment and validation of mucosal healing are needed. This is particularly true for Crohn's disease, where cross-sectional imaging of the bowel wall may be necessary to evaluate transmural rather than mucosal healing. Among those lines, it is important to point out that the mucosa only represents <15% of the thickness of the entire bowel wall. Thus, mucosal healing should be considered as an initial event in the suppression of inflammation of deeper layers of the bowel wall, rather than as a sign of complete healing of gut inflammation (figure 6). Both MRI and FDG-PET have been evaluated for the assessment of transmural bowel inflammation,174–177 although PET scanning is limited in part by radiation exposure and cost. These approaches need to be prospectively compared with ileocolonoscopy for assessing mucosal healing. Bowel ultrasound also has a potential role, since the ultrasound score after 3 months of steroid therapy accurately predicted clinical outcome at 15 months and concluded in a pilot study in 83 patients with severe UC,178 although the technique is highly operator-dependent.
Histological sections of the whole bowel wall in Crohn's disease (CD), ulcerative colitis (UC) and controls, illustrating that endoscopy and endomicroscopy provide information on healing of the mucosa (M) but not on the structure of the submucosa (S), the muscularis propria (MP) and the subserosa and serosa (SE). In contrast, ultrasound, FDG-PET and MRI can be used for imaging of inflammation of the whole bowel wall in inflammatory bowel disease. Marked inflammation of mucosa and submucosa in UC is shown. In contrast, transmural inflammation is seen in CD suggesting the relevance of cross-sectional imaging in this disease.
Biomarkers of mucosal inflammation, such as CRP, faecal calprotectin and lactoferrin have the potential to assess mucosal healing,179–181 although this still needs to be realised. In 77 patients with Crohn's disease, a threshold of 200 μg/g for a raised faecal calprotectin concentration showed a positive predictive value of 94%, and negative predictive value of 61% in predicting endoscopically active disease (CDEIS >3). Furthermore, normalisation of faecal calprotectin can predict favourable clinical outcomes in Crohn's disease after anti-TNF therapy.182 ,183 Rapid normalisation of CRP (within 12 weeks) also predicted mucosal healing after adalimumab in a study of 201 patients with Crohn's disease.184 Mucosal gene signatures are also being explored as markers of mucosal inflammation.185 ,186 Apart from antimicrobial peptides, expression of genes of the adaptive immune system may be markers of endoscopic and histological healing. Specifically, expression of osteoprotegerin, stanniocalcin-1, prostaglandin-endoperoxide synthase 2, interleukin 13 receptor α2 and interleukin 11 differed between anti-TNF responders and non-responders in UC, potentially discriminating between inflammation and mucosal healing. Further studies are warranted to evaluate the validity of serum markers or gene expression sets for assessing mucosal healing in IBD.
Conclusions
Therapeutic goals in Crohn's disease and UC have evolved to include mucosal healing as a measure of treatment efficacy. Mucosal healing is best defined as an absence of ulcers in Crohn's disease, since this has been shown to reduce the likelihood of clinical relapse, reduce the risk of surgery, and reduce hospitalisation.187 ,188 Definitions based on CDEIS (score <3 ‘complete endoscopic remission’, or <6 ‘endoscopic remission’) have yet to be shown to be associated with future disease behaviour. In UC, mucosal healing defined by a Mayo Clinic endoscopy subscore of 0 (normal, or inactive) at 8 weeks predicts symptom relief and steroid withdrawal at one year, while a subscore of 0 or 1 is associated with halving of the colectomy rate at 1 year.
Randomised, controlled trials have demonstrated that mucosal healing is attainable with some of the current arsenal of therapies, but there are substantial differences between Crohn's disease and UC in the effect of different drugs on mucosal healing (figure 4). In particular, while corticosteroids and 5-ASA promote mucosal healing in UC, neither achieve healing in Crohn's disease. This may be related to differences between transmural and mucosal inflammation, or the specific type of inflammation, since different responses of corticosteroids in experimental models of mucosal injury have been found.189 In any event, mucosal healing is emerging as an important therapeutic endpoint in clinical trials. It is transferring to clinical practice for predicting outcome in the following years in the postoperative assessment after ileocolic resection, early response to anti-TNF therapy in UC and the decision-making process before dose escalation, switching or stopping anti-TNF therapy. Attention needs to move from medium-term (1 year) outcomes to focus on the long term (>3 years) for diseases that currently last a lifetime.
Key messages
Mucosal healing in inflammatory bowel diseases (IBD)
-
Mucosal healing usually refers to endoscopic scores for assessment of disease activity.
-
The structural basis is an intact mucosal barrier including intestinal epithelial cells.
-
Future assessments of mucosal healing may relate to new endoscopic scores, serum markers and cross-sectional imaging techniques.
Clinical relevance of mucosal healing in IBD
-
Mucosal healing emerges as an important endpoint in clinical trials.
-
Anti-inflammatory drugs such as azathioprine and anti-TNF antibodies can induce mucosal healing.
-
Mucosal healing predicts lower hospitalisation rates, sustained clinical remission and resection-free survival.
-
Mucosal healing should be recognised by clinicians and healthcare providers as a key goal for IBD therapy.
Acknowledgments
The authors thank Professor H Neumann (University of Erlangen-Nürnberg, Germany) for endomicroscopic images and PD Michael Vieth (Pathology Department, Bayreuth, Germany) for histopathological images.
References
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
Footnotes
Competing interests MFN has served as an advisor to Pentax, Giuliani Pharma, MSD and Abbott.
Provenance and peer review Commissioned; externally peer reviewed.