Abstract
Objective: To assess the efficacy of pioglitazone treatment in comparison with that of acarbose treatment in patients with type 2 diabetes mellitus.
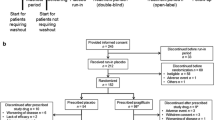
Participants and methods: In this randomized, parallel-group, open-label study patients were assigned to treatment with either pioglitazone (n = 129) or acarbose (n = 136). During a 1-week run-in patients commenced an individualized dietary regimen which was maintained throughout the study. Patients received the assigned study medication for 26 weeks. Serum glycosylated hemoglobin (HbA1c) levels, insulin resistance and lipid profiles were determined at baseline and at endpoint.
Results: Mean HbA1c was reduced from 8.98 ± 1.20% to 7.82 ± 1.95% with pioglitazone treatment and from 9.03 ± 1.32% to 8.55 ± 1.96% with acarbose treatment during the 26-week study. The change from baseline to endpoint was significantly greater for pioglitazone compared with acarbose when analyzed for all patients (p < 0.001) and for those who had (p = 0.009) or had not (p < 0.001) received previous medication for diabetes mellitus. Compared with acarbose, pioglitazone produced a significantly greater decrease in fasting glucose, insulin and insulin resistance (p < 0.001 for each). Triglycerides were decreased by 71.1 ± 184.1 mg/dl with pioglitazone compared with 38.1 ± 171.3 mg/dl with acarbose (p = 0.001 for difference between groups). High density lipoprotein (HDL)-cholesterol level was increased by 7.8 ± 10.2 mg/dl with pioglitazone compared with a decrease of 0.8 ± 24.1 mg/dl with acarbose (p < 0.001). While serum low density lipoprotein (LDL)-cholesterol levels remained unchanged with both treatment regimens, the decrease from baseline in very low density lipoprotein (VLDL)-cholesterol was significantly greater with pioglitazone than with acarbose (p < 0.04). Pioglitazone decreased systolic blood pressure by 5.6 ± 17.7mm Hg compared with a 0.4 ± 18.4mm Hg increase during acarbose treatment (p < 0.001). Pioglitazone caused a significantly greater decrease compared with acarbose in serum levels of γ-glutamyl aminotransferase (p < 0.001) and alanine aminotransferase (p = 0.004).
Conclusions: Six months of pioglitazone treatment decreased insulin resistance and improved glycemic control to a significantly greater extent than acarbose treatment. Pioglitazone was also associated with a significantly improved lipid profile, suggesting a reduction in risk of coronary heart disease.







Similar content being viewed by others
Notes
The use of tradenames is for product identification purposes only and does not imply endorsement.
References
DECODE Study Group on behalf of the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med 2001; 161: 617–24
Gu K, Cowie CC, Harris MI. Diabetes and the decline in heart disease mortality in US adults. JAMA 1999; 281: 1291–7
Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339: 229–34
Stamler J, Vaccaro O, Neaton JD, et al. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993; 16: 434–44
UK Prospective Diabetes Study Group. Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–53
Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35). BMJ 2000; 321: 405–12
American Diabetes Association. Consensus development conference on insulin resistance. Diabetes 1998; 21: 310–4
DeFronzo RA. The triumverate: beta-cell, muscle and liver: a collusion responsible for NIDDM. Diabetes 1988; 37: 667–87
Haffner SM, Mykkanen L, Festa A, et al. Insulin-resistant prediabetic subjects have more atherogenic risk factors than insulin-sensitive prediabetic subjects: implications for preventing coronary heart disease during the prediabetic state. Circulation 2000; 101: 975–80
Weyer C, Bogardus C, Mott DM, et al. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 1999; 104: 787–94
Groop LC. Insulin resistance: the fundamental trigger of type 2 diabetes. Diabetes Obes Metab 1999; 1 Suppl. 1: S1–7
DeFronzo RA, Ferrannini E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidaemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991; 14: 173–94
Kuller LH, Velentgas P, Barzillay J, et al. Diabetes mellitus: subclinical cardiovascular disease and risk of incident cardiovascular disease and all cause mortality. Arterioscler Thromb Vasc Biol 2000; 29: 823–9
Cullen P, von Eckardstein A, Souris S, et al. Dyslipidaemia and cardiovascular risk in diabetes. Diabetes Obes Metab 1999; 1: 189–98
Laakso M, Sarlund H, Mykkanen L. Insulin resistance is associated with lipid and lipoprotein abnormalities in subjects with varying degrees of glucose intolerance. Atherosclerosis 1990; 10: 223–31
Holman RR, Cull CA, Turner RC. A randomised double-blind trial of acarbose in type 2 diabetes shows improved glycaemic control over 3 years (UKPDS 44). Diabetes Care 1999; 22: 960–4
Scorpiglione N, Belfiglio M, Carinci F, et al. The effectiveness, safety and epidemiology of the use of acarbose in the treatment of patients with type II diabetes mellitus: a model of medicine-based evidence. Eur J Clin Pharmacol 1999; 55: 239–49
Otto C, Lehrke M, Göke B. Novel insulin sensitizers: pharmacogenomic aspects. Pharmacogenomics 2002; 3: 1–18
Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev 1999; 20: 649–88
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–9
Salman S, Salman F, Satman I, et al. Comparison of acarbose and gliclazide as first-line agents in patients with type 2 diabetes. Curr Med Res Opin 2001; 16: 296–306
Shaw JT, Purdie DM, Neil HA, et al. The relative risks of hyperglycaemia, obesity and dyslipidaemia in the relatives of patients with type II diabetes. Diabetologia 1999; 42: 24–7
Chitturi S, Abeygunasekera S, Farrell GC, et al. NASH and insulin resistance: insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology 2002; 35: 373–9
Reddy JK. Nonalcoholic steatosis and steatohepatitis: III. peroxisomal beta-oxidation, PPAR alpha, and steatohepatitis. Am J Physiol 2001; 281: G1333–9
Baumgartner-Parzer SM, Waldhäusl WK. The endothelium as a metabolic and endocrine organ: its relation with insulin resistance. Exp Clin Endocrinol Diabetes 2001; 109 Suppl. 2: S166–79
Acknowledgements
This study was funded by Takeda Pharma, Germany. As a member of the advisory board of Takeda Pharma, Professor Goke participated in this study as principal investigator.
Author information
Authors and Affiliations
Consortia
Rights and permissions
About this article
Cite this article
Göke, B., German Pioglitazone Study Group. Improved Glycemic Control and Lipid Profile in a Randomized Study of Pioglitazone Compared with Acarbose in Patients with Type 2 Diabetes Mellitus. Mol Diag Ther 1, 329–336 (2002). https://doi.org/10.2165/00024677-200201050-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00024677-200201050-00005