Abstract
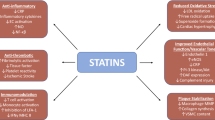
HMG-CoA reductase is the rate-limiting enzyme for cholesterol synthesis and its inhibition exerts profound effects on cellular metabolism. Inhibitors of this enzyme are used in clinical practice to lower plasma cholesterol levels and are commonly collectively referred to as ‘statins’. A number of in vitro, in vivo animal, and clinical studies suggest that properties of statins other than cholesterol lowering may be of biological importance. These diverse properties are often referred to as ‘pleiotropic’ and suggest that statins may affect a number of diseases of ageing. In this article we review the biological plausibility and clinical evidence of a role for statins in modulating two diseases of ageing: osteoporosis and dementia (including Alzheimer’s disease). In both diseases, there is a sound cellular and laboratory basis for a plausible therapeutic effect of statins. In the case of osteoporosis, there are conflicting data regarding clinical benefit, with both negative and positive results reported. In particular, secondary analyses of randomised, controlled studies have shown no reduction of fracture risk by statins. In the case of dementia there are fewer clinical studies but there is clear anticipated benefit in macrovascular dementias attributable to statin-mediated reduction of the risk of stroke. Overall, there are a lack of prospective, placebo-controlled, randomised data testing statins and modulation of the risk of osteoporosis-related fracture or of clinical dementia, where these are primary outcomes. Until such data are available, the use of statins appears promising but cannot be recommended as a primary therapeutic modality for either condition.


Similar content being viewed by others
References
Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science 1986; 232(4746): 34–47
Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344 3–9
Sacks FM, Tonkin AM, Shepherd J, et al. Effect of pravastatin on coronary disease events in subgroups defined by coronary risk factors: the Prospective Pravastatin Pooling Project. Circulation. 2000; 102(16): 1893–900
Watts GF, Burke V. Lipid-lowering trials in the primary and secondary prevention of coronary heart disease: new evidence, implications and outstanding issues. Curr Opin Lipidol 1996; 7(6): 341–55
Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol 2001; 21(11): 1712–9
Gotto AM Jr, Farmer JA. Pleiotropic effects of statins: do they matter? Curr Opin Lipidol 2001; 12(4): 391–4
Comparato C, Altana C, Bellosta S, et al. Clinically relevant pleiotropic effects of statins: drug properties or effects of profound cholesterol reduction? Nutr Metab Cardiovasc Dis 2001; 11(5): 328–43
Rosenson RS, Tangney CC. Antiatherothrombotic properties of statins: implications for cardiovascular event reduction. JAMA 1998; 279(20): 1643–50
LaRosa JC. Pleiotropic effects of statins and their clinical significance. Am J Cardiol 2001; 88(3): 291–3
Aikawa M, Rabkin E, Okada Y, et al. Lipid lowering by diet reduces matrix metalloproteinase activity and increases collagen content of rabbit atheroma: a potential mechanism of lesion stabilization. Circulation 1998; 97(24): 2433–44
Yamamoto A, Harada-Shiba M, Kawaguchi A, et al. Apheresis technology for prevention and regression of atherosclerosis. Ther Apher 2001; 5(4): 221–5
Mellwig KP, Baller D, Gleichmann U, et al. Improvement of coronary vasodilatation capacity through single LDL apheresis. Atherosclerosis 1998; 139(1): 173–8
Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature 1990; 343(6257): 425–30
Glomset JA, Farnsworth CC. Role of protein modification reactions in programming interactions between ras-related GTPases and cell membranes. Annu Rev Cell Biol 1994; 10: 181–205
Lennernas H, Fager G. Pharmacodynamics and pharmacokinetics of the HMG-CoA reductase inhibitors: similarities and differences. Clin Pharmacokinet 1997; 32(5): 403–25
Hamelin BA, Turgeon J. Hydrophilicity/lipophilicity: relevance for the pharmacology and clinical effects of HMG-CoA reductase inhibitors. Trends Pharmacol Sci 1998; 19(1): 26–37
Bonn V, Cheung RC, Chen B, et al. Simvastatin, an HMG-CoA reductase inhibitor, induces the synthesis and secretion of apolipoprotein AI in HepG2 cells and primary hamster hepatocytes. Atherosclerosis 2002; 163(1): 59–68
Corsini A, Bellosta S, Baetta R, et al. New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Ther 1999; 84(3): 413–28
Chong PH, Seeger JD, Franklin C. Clinically relevant differences between the statins: implications for therapeutic selection. Am J Med 2001; 111(5): 390–400
Warwick MJ, Dane AL, Schneck DW, et al. Single- and multiple-dose pharmacokinetics and safety of the new HMG CoA-reductase inhibitor. Atherosclerosis 2000; 151: 39, Abstract P19: W6
Martin PD, Dane AL, Schneck DE, et al. Disposition of new HMG-CoA reductase inhibitor ZD4522 following dosing in healthy subjects [abstract 48]. J Clin Pharmacol 2000; 40: 1056
Buckett L, Ballard P, Davidson R, et al. Selectivity of ZD4522 for inhibition of cholesterol synthesis in hepatic versus non-hepatic cells. Atherosclerosis 2000; 151: 41
Sabia H, Prasad P, Smith HT, et al. Safety, tolerability, and pharmacokinetics of an extended-release formulation of fluvastatin administered once daily to patients with primary hypercholesterolemia. J Cardiovasc Pharmacol 2001; 37(5): 502–11
McCormick AD, McKillop D, Butters CJ, et al. ZD4522-An HMG CoA reductase inhibitor free of metabolically mediated drug interaction: metabolic studies in human in vitro systems. Chicago (IL): American College of Clinical Pharmacology, 2000
Ridker PM, Rifai N, Pfeffer MA, et al. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation 1999; 100(3): 230–5
Le Couteur DG, Cogger VC, Markus AMA, et al. Pseudocapiilarization and associated energy limitation in the aged rat liver. Hepatology 2001; 33: 537–43
Davidson MH. Rosuvastatin: a highly efficacious statin for the treatment of dyslipidaemia. Expert Opin Investig Drugs. 2002; 11(1): 125–41
Lips P. Epidemiology and predictors of fractures associated with osteoporosis. Am J Med 1997; 103(2A): 3S–8S; discussion 8S-11S
Johnell O. The socioeconomic burden of fractures: today and in the 21st century. Am J Med 1997; 103(2A): 20S–25S; discussion 25S-6S
van Daele PL, Seibel MJ, Burger H, et al. Case-control analysis of bone resorption markers, disability, and hip fracture risk: the Rotterdam study. BMJ 1996; 312(7029): 482–3
Garnero P, Sornay-Rendu E, Chapuy MC, et al. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res 1996; 11(3): 337–49
Garnero P, Sornay-Rendu E, Claustrat B, et al. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res 2000; 15(8): 1526–36
Tintut Y, Demer LL. Recent advances in multifactorial regulation of vascular calcification. Curr Opin Lipidol 2001; 12(5): 555–60
Burnett JR, Vasikaran SD. Cardiovascular disease and osteoporosis: is there a link between lipids and bone? Ann Clin Biochem 2002; 39 (Pt 3): 203–10
Hak AE, Pols HA, van Hemert AM, et al. Progression of aortic calcification is associated with metacarpal bone loss during menopause: a population-based longitudinal study. Arterioscler Thromb Vasc Biol 2000; 20(8): 1926–31
Parhami F, Morrow AD, Balucan J, et al. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiationL: a possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol 1997; 17(4): 680–7
Parhami F, Tintut Y, Ballard A, et al. Leptin enhances the calcification of vascular cells: artery wall as a target of leptin. Circ Res 2001; 88(9): 954–60
Pfeilschifter J, Koditz R, Pfohl M, et al. Changes in pro-inflammatory cytokine activity after menopause. Endocr Rev 2002; 23(1): 90–119
Ormarsdottir S, Ljunggren O, Mallmin H, et al. Inverse relationship between circulating levels of leptin and bone mineral density in chronic liver disease. J Gastroenterol Hepatol 2001; 16(12): 1409–14
Amling M, Pogoda P, Beil FT, et al. Central control of bone mass: brainstorming of the skeleton. Adv Exp Med Biol 2001; 496: 85–94
Wallace AM, McMahon AD, Packard CJ, et al. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS). Circulation 2001; 104(25): 3052–6
Crane SK. Genetic control of bone remodelling-insights from a rare disease. N Engl J Med 2002; 347: 210–2
Chan GK, Duque G. Age-related bone loss: old bone, new facts. Gerontology 2002; 48(2): 62–71
Treloar V. Bisphosphonates and osteoporosis. N Engl J Med 2002; 346(26): 2088–9
Maricic M, Adachi JD, Sarkar S, et al. Early effects of raloxifene on clinical vertebral fractures at 12 months in postmenopausal women with osteoporosis. Arch Intern Med 2002; 162(10): 1140–3
Rosen CJ, Bilezikian JP. Clinical review 123: anabolic therapy for osteoporosis. J Clin Endocrinol Metab 2001; 86(3): 957–64
Delmas PD. Treatment of postmenopausal osteoporosis. Lancet 2002; 359(9322): 2018–26
Mundy G, Garrett R, Harris S, et al. Stimulation of bone formation in vitro and in rodents by statins. Science 1999; 286 (5446): 1946–9
Sugiyama M, Kodama T, Konishi K, et al. Compactin and simvastatin, but not pravastatin, induce bone morphogenetic protein-2 in human osteosarcoma cells. Biochem Biophys Res Commun. 2000; 271(3): 688–92
van Beek E, Lowik C, van der Pluijm G, et al. The role of geranylgeranylation in bone resorption and its suppression by bisphosphonates in fetal bone expiants in vitro: a clue to the mechanism of action of nitrogen-containing bisphosphonates. J Bone Miner Res 1999; 14(5): 722–9
Luckman SP, Hughes DE, Coxon FP, et al. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res 1998; 13(4): 581–9
Chan MH, Mak TW, Chiu RW, et al. Simvastatin increases serum osteocalcin concentration in patients treated for hyper-cholesterolaemia. J Clin Endocrinol Metab 2001; 86(9): 4556–9
Chung YS, Lee MD, Lee SK, et al. HMG-CoA reductase inhibitors increase BMD in type 2 diabetes mellitus patients. J Clin Endocrinol Metab 2000; 85(3): 1137–42
Hsia J, Morse M, Levin V. Effect of simvastatin on bone markers in osteopenic women: a placebo-controlled, dose-ranging trial [ISRCTN85429598]. BMC Musculoskelet Disord 2002; 3(1): 7
Edwards CJ, Hart DJ, Spector TD. Oral statins and increased bone mineral density in post menopausal women. Lancet 2000; 355: 2218–9
Watanabe S, Fukumoto S, Takeuchi Y, et al. Effects of 1 year treatment with fluvastatin or pravastatin on bone. Am J Med 2001; 110: 584–7
Chan KA, Andrade SE, Boles M, et al. Inhibitors of hydroxymethylglutaryl-coenzyme A reductase and risk of fracture among older women. Lancet 2000; 355(9222): 2185–8
Meier CR, Schlienger RG, Kraenzlin ME, et al. HMG-CoA reductase inhibitors and the risk of fractures. JAMA 2000; 283(24): 3205–10
van Staa TP, Wegman S, de Vries F, et al. Use of statins and risk of fractures. JAMA 2001; 285(14): 1850–5
Wang PS, Solomon DH, Mogun H, et al. HMG-CoA reductase inhibitors and the risk of hip fractures in elderly patients. JAMA 2000; 283(24): 3211–6
Bauer DC, Mundy GR, Jamal SA, et al. Statin use, bone mass and fracture: an analysis of two prospective studies [abstract]. J Bone Miner Res 1999; 14: S179
Reid IR, Hague W, Emberson J, et al. Effect of pravastatin on frequency of fracture in the LIPID study: secondary analysis of a randomised control trial. Lancet 2001; 357: 509–12
Pedersen TR, Kjekshus J. Statin drugs and the risk of fracture. 4S Study Group. JAMA 2000; 284(15): 1921–2
Heart Protection Study Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20356 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360: 7–22
Knopman DS. An overview of common non-Alzheimer dementias. Clin Geriatr Med 2001; 17: 281–301
Evans DA, Funkenstein HH, Albert MS, et al. Prevalence of Alzheimer’s disease in a community population of older persons: higher than previously reported. JAMA 1989; 262(18): 2551–6
Kril JJ, Halliday GM. Alzheimer’s disease: its diagnosis and pathogenesis. Int Rev Neurobiol 2001; 48: 167–217
Esiri MM, Wilcock GK, Morris JH. Neuropathological assessment of the lesions of significance in vascular dementia. J Neurol Neurosurg Psychiatry 1997; 63: 749–53
Jagust W. Untangling vascular dementia. Lancet 2001; 358: 2097–8
Henon H, Durieu I, Guerouaou D, et al. Poststroke dementia: incidence and relationship to prestroke cognitive decline. Neurology 2001; 57(7): 1216–22
Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 1996; 335 (14): 1001–9
White HD, Simes RJ, Anderson NE, et al. Pravastatin therapy and the risk of stroke. N Engl J Med. 2000; 343(5): 317–26
Yip CM, Elton EA, Darabie AA, et al. Cholesterol, a modulator of membrane-associated Abeta-fibrillogenesis and neurotoxicity. J Mol Biol 2001; 311(4): 723–34
Mori T, Paris D, Town T, et al. Cholesterol accumulates in senile plaques of Alzheimer disease patients and in transgenic APP(SW) mice. J Neuropathol Exp Neurol 2001; 60(8): 778–85
Kakio A, Nishimoto SI, Yanagisawa K, et al. Cholesterol-dependent formation of GM1 ganglioside-bound amyloid beta-protein, an endogenous seed for Alzheimer amyloid. J Biol Chem 2001; 276(27): 24985–90
Sparks DL, Scheff SW, Hunsaker JC 3rd, et al. Induction of Alzheimer-like beta-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp Neurol 1994; 126(1): 88–94
Sparks DL. Coronary artery disease, hypertension, ApoE, and cholesterol: a link to Alzheimer’s disease? Ann N Y Acad Sci 1997; 826: 128–46
Poirier J, Delisle MC, Quirion R, et al. Apolipoprotein E4 allele as a predictor of cholinergic deficits and treatment outcome in Alzheimer disease. Proc Natl Acad Sci USA 1995; 92(26): 12260–4
Petrovitch H, White LR, Izmirilian G, et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Honolulu-Asia aging Study. Neurobiol Aging 2000; 21(1): 57–62
Bruce DG, Harrington N, Davis WA, et al. Dementia and its associations in type 2 diabetes mellitus: the Fremantle Diabetes Study. Diabetes Res Clin Pract 2001; 53(3): 165–72
Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ 2001; 322(7300): 1447–51
Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipidol 2001; 12(2): 105–12
Papassotiropoulos A, Lutjohann D, Bagli M, et al. 24S-hydroxycholesterol in cerebrospinal fluid is elevated in early stages of dementia. J Psychiatr Res 2002; 36(1): 27–32
Locatelli S, Lutjohann D, Schmidt HH, et al. Reduction of plasma 24S-hydroxycholesterol (cerebrosterol) levels using high-dosage simvastatin in patients with hypercholesterolemia: evidence that simvastatin affects cholesterol metabolism in the human brain. Arch Neurol 2002; 59(2): 213–6
Fassbender K, Simons M, Bergmann C, et al. Simvastatin strongly reduces levels of Alzheimer’s disease beta — amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci USA 2001; 98(10): 5856–61
Endres M, Laufs U, Huang Z, et al. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci USA 1998; 95(15): 8880–5
Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem 1998; 273(37): 24266–71
Carr DB, Goate A, Phil D, et al. Current concepts in the pathogenesis of Alzheimer’s disease. Am J Med 1997; 103(3A): 3S–10S
Breitner JC, Gau BA, Welsh KA, et al. Inverse association of anti-inflammatory treatments and Alzheimer’s disease: initial results of a co-twin control study. Neurology 1994; 44(2): 227–32
Halliday GM, Shepherd CE, McCann H, et al. Effect of anti-inflammatory medications on neuropathological findings in Alzheimer disease. Arch Neurol 2000; 57(6): 831–6
Palinski W. Immunomodulation: a new role for statins? Nat Med 2000; 6(12): 1311–2
Kwak B, Mulhaupt F, Myit S, et al. Statins as a newly recognized type of immunomodulator. Nat Med 2000; 6(12): 1399–402
Simons M, Keller P, De Strooper B, et al. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc Natl Acad Sci USA 1998; 95(11): 6460–4
Kilsdonk EP, Yancey PG, Stoudt GW, et al. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem 1995; 270(29): 17250–6
Kritharides L, Kus M, Brown AJ, et al. Hydroxypropyl-beta-cyclodextrin-mediated efflux of 7-ketocholesterol from macrophage foam cells. J Biol Chem 1996; 271(44): 27450–5
Wolozin B, Kellman W, Ruosseau P, et al. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol 2000; 57(10): 1439–43
Tsuji A, Saheki A, Tamai I, et al. Transport mechanism of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors at the blood-brain barrier. J Pharmacol Exp Ther 1993; 267 (3): 1085–90
Saheki A, Terasaki T, Tamai I, et al. In vivo and in vitro blood-brain barrier transport of 3-hydroxy-3- methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors. Pharm Res 1994; 11(2): 305–11
Quion JA, Jones PH. Clinical pharmacokinetics of pravastatin. Clin Pharmacokinet 1994; 27(2): 94–103
Wolozin B. Letter. Arch Neurol 2001; 58: 1023
Jick H, Zornberg GL, Jick SS, et al. Statins and the risk of dementia. Lancet 2000; 356(9242): 1627–31
Friedhoff LT, Cullen EI, Geoghagen NS, et al. Treatment with controlled-release lovastatin decreases serum concentrations of human beta-amyloid (A beta) peptide. Int J Neuropsychopharmacol 2001; 4(2): 127–30
Muldoon MF, Barger SD, Ryan CM, et al. Effects of lovastatin on cognitive function and psychological well-being. Am J Med 2000; 108(7): 538–46
Shepherd J, Blauw GJ, Murphy MB, et al. The design of a prospective study of Pravastatin in the Elderly at Risk (PROSPER). PROSPER Study Group. PROspective Study of Pravastatin in the Elderly at Risk. Am J Cardiol 1999; 84(10): 1192–7
Acknowledgements
Professors J. Kril, D. LeCouteur and M. Seibel are thanked for many helpful discussions.
Dr. Kritharides is a member of the Australian Scientific Advisory Committee for rosuvastatin (Astra-Zeneca), and has given invited presentations at scientific symposia convened or supported by Bristol Myer Squibb, Astra Zeneca, and Merck Sharp and Dohme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Waldman, A., Kritharides, L. The Pleiotropic Effects of HMG-CoA Reductase Inhibitors. Drugs 63, 139–152 (2003). https://doi.org/10.2165/00003495-200363020-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200363020-00002