Abstract
The maturation of organ systems during fetal life and childhood exerts a profound effect on drug disposition. The maturation of drug-metabolising enzymes is probably the predominant factor accounting for age-associated changes in non-renal drug clearance. The group of drug-metabolising enzymes most studied are the cytochrome P450 (CYP) superfamily. The CYP3A subfamily is the most abundant group of CYP enzymes in the liver and consists of at least 3 isoforms: CYP3A4, 3A5 and 3A7. Many drugs are mainly metabolised by the CYP3A subfamily. Therefore, maturational changes in CYP3A ontogeny may impact on the clinical pharmacokinetics of these drugs.
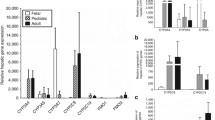
CYP3A4 is the most abundantly expressed CYP and accounts for approximately 30 to 40% of the total CYP content in human adult liver and small intestine. CYP3A5 is 83% homologous to CYP3A4, is expressed at a much lower level than CYP3A4 in the liver, but is the main CYP3A isoform in the kidney. CYP3A7 is the major CYP isoform detected in human embryonic, fetal and newborn liver, but is also detected in adult liver, although at a much lower level than CYP3A4. Substrate specificity for the individual isoforms has not been fully elucidated. Because of large interindividual differences in CYP3A4 and 3A5 expression and activity, genetic polymorphisms have been suggested. However, although some gene mutations have been identified, the impact of these mutations on the pharmacokinetics of CYP3A substrates has to be established.
Ontogeny of CYP3A activity has been studied in vitro and in vivo. CYP3A7 activity is high during embryonic and fetal life and decreases rapidly during the first week of life. Conversely, CYP3A4 is very low before birth but increases rapidly thereafter, reaching 50% of adult levels between 6 and 12 months of age. During infancy, CYP3A4 activity appears to be slightly higher than that of adults. Large interindividual variations in CYP3A5 expression and activity were observed during all stages of development, but no apparent developmental pattern of CYP3A5 activity has been identified to date.
Profound changes occur in the activity of CYP3A isoforms during all stages of development. These changes have, in many instances, proven to be of clinical significance when treatment involves drugs that are substrates, inhibitors or inducers of CYP3A. Investigators and clinicians should consider the impact of ontogeny on CYP3A in both pharmacokinetic study design and data interpretation, as well as when prescribing drugs to children.
Similar content being viewed by others
References
Morselli PL, Franco-Morselli R, Bossi L. Clinical pharmacokinetics in newboms and infants: age-related differences and therapeutic implications. Clin Pharmacokinet 1980; 5: 485–527.
Anderson BJ, McKee AD, Holford NHG. Size, myths and the clinical pharmacokinetics of analgesia in paediatric patients. Clin Pharmacokinet 1997; 33 (5): 313–27.
Shimada T, Yamazaki H, Mimura M, et al. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther 1994; 270 (1): 414–23.
Nelson DR, Koymans L, Kamataki T, et al. P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics 1996; 6 (1): 1–42.
Lacroix D, Sonnier M, Moncion A, et al. Expression of CYP3A in the liver evidence that the shift between CYP3A7 and CYP3A4 occurs immediately afterbirth. Eur JBiochem 1997; 247: 625–34.
Cresteil T. Onset of xenobiotic metabolism in children: toxicological implications. Food Addit Contain 1998; 15 (S): 45–51.
Yang H-YL, Lee QP, Rettie AE, et al. Functional cytochrome 3A isoforms in human embryonic tissues: expression during organogenesis. Mol Pharmacol 1994; 46: 922–8.
Rich KJ, Boobis AR. Expression and inducibility of P450 enzymes during liver ontogeny. Microsc Res Tech 1997; 39 (5): 424–35.
Tateishi T, Nakura H, Asoh M, et al. A comparison of hepatic cytochrome P450 protein expression between infancy and postinfancy. Life Sci 1997; 61 (26): 2567–74.
Leeder JS, Kearns GL. Pharmacogenetics in pediatrics. Pediatr Clin North Am 1997; 44 (1): 55–77.
Wrighton SA, Stevens JC. The human hepatic cytochromes P450 involved in drug metabolism. Crit Rev Toxicol 1992; 22 (1): 1–21.
Batt AM, Magdalou J, Vincent-Viry M, et al. Drug metabolizing enzymes related to laboratory medicine: cytochromes P-450 and UDP-glucuronosyltransferases [review]. Clin Chim Acta 1994; 226 (2): 171–90.
Wrighton SA, VandenBranden M, Ring BJ. The human drug metabolizing cytochromes P450. J Pharmacokinet Biopharm 1996; 24 (5): 461–73.
Watkins B, Wrighton SA, Schuetz EG, et al. Identification of glucocorticoid-inducible cytochromes P-450 in the intestinal mucosa of rats and man. J Clin Invest 1987; 80 (4): 1029–36.
McKinnon RA, Burgess WM, Hall PM, et al. Characterisation of CYP3A gene subfamily expression in human gastrointestinal tissues. Gut 1995; 36 (2): 259–67.
Kivisto KT, Bookjans G, Fromm MF, et al. Expression of CYP3A4, CYP3A5 and CYP3A7 in human duodenal tissue. Br J Clin Pharmacol 1996; 42 (3): 387–9.
De Waziers I, Cugnenc PH, Yang CS, et al. Cytochrome P 450 isoenzymes, epoxide hydrolase and glutathione transferases in rat and human hepatic and extrahepatic tissues. J Pharmacol Exp Ther 1990; 253 (1): 387–94.
Inoue K, Inazawa J, Nakagawa H, et al. Assignment of the human cytochrome P-450 nifedipine oxidase gene (CYP3A4) to chromosome 7 at band q22.1 by fluorescence in situ hybridization. Jpn J Human Genet 1992; 37: 133–8.
Komori M, Nishio K, Ohi H, et al. Molecular cloning and sequence analysis of cDNA containing the entire coding region for human fetal liver cytochrome P-450. J Biochem (Tokyo) 1989; 105 (2): 161–3.
Kolars JC, Lown KS, Schmiedlin-Ren P, et al. CYP3A gene expression in human gut epithelium. Pharmacogenetics 1994; 4: 247–59.
Ratanasavanh D, Beaune P, Morel F, et al. Intralobular distribution and quantitation of cytochrome P-450 enzymes in human liver as a function of age. Hepatology 1991; 13 (6): 1142–51.
Hashimoto H, Toide K, Kitamura R, et al. Gene structure of CYP3A4, an adult-specific form of cytochrome P450 in human livers, and its transcriptional control. Eur J Biochem 1993; 218: 585–95.
Shimada T, Guengerich F. Evidence for cytochrome P-450NF, the nifedipine oxidase, being the principal enzyme involved in the bioactivation of aflatoxins in human liver. Proc Natl Acad Sci U S A 1989; 86 (2): 462–5.
Michalets EL. Update: clinically significant cytochrome P-450 drug interactions. Pharmacotherapy 1998; 18 (1): 84–112.
Kearns GL. Pharmacogenetics and development: are infants and children at increased risk for adverse outcomes? Curr Opin Pediatr 1995; (7): 220–33.
Schuetz EG, Schuetz JD, Strom SC, et al. Regulation of human liver cytochromes P-450 in family 3A in primary and continuous culture of human hepatocytes. Hepatology 1993; 18 (5): 1254–62.
Wrighton SA, Ring BJ, Watkins PB, et al. Identification of a polymorphically expressed member of the human cytochrome P-450III family. Mol Pharmacol 1989; 36 (1): 97–105.
Aoyama T, Yamano S, Waxman DJ, et al. Cytochrome P-450 hPCN3, a novel cytochrome P-450 IIIA gene product that is differentially expressed in adult human liver: cDNA and deduced amino acid sequence and distinct specificities of cDNA-expressed hPCN1 and hPCN3 for the metabolism of steroid hormones and cyclosporine. J Biol Chem 1989; 264 (18): 10388–95.
Transon C, Lecoeur S, Leemann T, et al. Interindividual variability in catalytic activity and immunoreactivity of three major human liver cytochrome P450 isozymes. Eur J Clin Pharmacol 1996; 51 (1): 79–85.
Jounaidi Y, Hyrailles V, Gervot L, et al. Detection of CYP3A5 allelic variant: a candidate for the polymorphic expression of the protein? Biochem Biophys Res Commun 1996; 221 (2): 466–70.
Gorski JC, Hall SD, Jones DR, et al. Regioselective biotransformation of midazolam by members of the human cytochrome P450 3A (CYP3A) subfamily. Biochem Pharmacol 1994; 47 (9): 1643–53.
Wrighton SA, Brian WR, Sari MA, et al. Studies on the expression and metabolic capabilities of human liver cytochrome P450IIIA5 (HLp3). Mol Pharmacol 1990; 38 (2): 207–13.
Gillam EM, Guo Z, Ueng YF, et al. Expression of cytochrome P450 3A5 in Escherichia coli: effects of 5′ modification, purification, spectral characterization, reconstitution conditions, and catalytic activities [published erratum appears in Arch Biochem Biophys 1995 Apr 20; 318 (2): 498]. Arch Biochem Biophys 1995; 317 (2): 374–84.
Ohmori S, Nakasa H, Asanome K, et al. Differential catalytic properties in metabolism of endogenous and exogenous substrates among CYP3A enzymes expressed in COS-7 cells. Biochim Biophys Acta 1998; 1380 (3): 297–304.
Kitada M, Kamataki T, Itahashi K, et al. Purification and properties of cytochrome P-450 from homogenates of human fetal livers. Arch Biochem Biophys 1985; 241 (1): 275–80.
Gotschall RR, Marcucci K, Leeder JS, et al. Cisapride (CIS) biotransformation: not all CYP3A are created equal. Clin Pharmacol Ther 1999; 65 (2): 127A.
Kitada M, Kamataki T, Itahashi K, et al. P-450 HFLa, a form of cytochrome P-450 purified from human fetal livers, is the 16 alpha-hydroxylase of dehydroepiandrosterone 3-sulfate. J Biol Chem 1987; 262 (28): 13534–7.
Hashimoto H, Nakagawa T, Yokoi T, et al. Fetus-specific CYP3A7 and adult-specific CYP3A4 expressed in Chinese hamster CHL cells have similar capacity to activate carcinogenic mycotoxins. Cancer Res 1995; 55 (4): 787–91.
Schuetz JD, Beach DL, Guzelian PS. Selective expression of cytochrome P450 CYP3A mRNAs in embryonic and adult human live. Pharmacogenetics 1994; 4: 11–20.
Fahr A. Cyclosporin clinical pharmacokinetics. Clin Pharmacokinet 1993; 24 (6): 472–95.
Horsmans Y, Desager JP, Harvengt C. Absence of CYP3A genetic polymorphism assessed by urinary excretion of 6 beta-hydroxycortisol in 102 healthy subjects on rifampicin. Pharmacol Toxicol 1992; 71 (4): 258–61.
Jones DR, Gorski JC, Haehner DB, et al. Determination of cytochrome p450 3a4/5 activity in vivo with dextromethorphan n-demethylation. Clin Pharmacol Ther 1996; 60 (4): 374–84.
Jacqz-Aigrain E, Daoud P, Burtin P, et al. Placebo-controlled trial of midazolam sedation in mechanically ventilated newborn babies. Lancet 1994; 344: 646–50.
Paine MF, Shen DD, Kunze KL, et al. First-pass metabolism of midazolam by the human intestine. Clin Pharmacol Ther 1996; 60 (1): 14–24.
Watkins PB, Murray SA, Winkelman LG, et al. Erythromycin breath test as an assay of glucocorticoid-inducible liver cytochromes P-450. J Clin Invest 1989; 83: 688–97.
Thummel KE, Shen DD, Podoll TD, et al. Use of midazolam as a human cytochrome P450 3A probe: II. Characterization of inter- and intra individual hepatic CYP3A variability after liver transplantation. J Pharmacol Exp Ther 1994; 271: 557–66.
Kronbach T, Mathys D, Umeno M, et al. Oxidation of midazolam and triazolam by human liver cytochrome P450IIIA4. Mol Pharmacol 1989; 36 (1): 89–96.
Kashuba AD, Dertino Jr JS, Rocci Jr ML, et al. Quantification of 3-month intraindividual variability and the influence of sex and menstrual cycle phase on CYP3A activity as measured by phenotyping with intravenous midazolam. Clin Pharmacol Ther 1998; 64 (3): 269–77.
Tateishi T, Watanabe M, Moriya H, et al. No ethnic difference between Caucasian and Japanese hepatic samples in the expression frequency of CYP3A5 and CYP3A7 proteins. Biochem Pharmacol 1999; 57 (8): 935–9.
Chavez-Teyes L, Castaneda-Hernandez G, Flores-Murrieta FJ. Pharmacokinetics of midazolam in Mexicans. Evidence for interethnic variability. Clin Drug Invest 1999; 17 (3): 233–9.
Watkins PB. Noninvasive tests of CYP3A enzymes. Pharmacogenetics 1994; 4: 171–84.
Lampen A, Christians U, Bader A, et al. Drug interactions and interindividual variability of ciclosporin metabolism in the small intestine. Pharmacology 1996; 52 (3): 159–68.
Christians U, Bleck JS, Lampen A, et al. Are cytochrome P450 3A enzymes in the small intestine responsible for different cyclosporine metabolite patterns in stable male and female renal allograft recipients after co-administration of diltiazem? Transplant Proc 1996; 28 (4): 2159–61.
Rebbeck TR, Jaffe JM, Walker AH, et al. Modification of clinical presentation of prostate tumors by a novel genetic variant in CYP3A4. J Natl Cancer Inst 1998; 90 (16): 1225–9.
Felix CA, Walker AH, Lange BJ, et al. Association of CYP3A4 genotype with treatment-related leukemia. Proc Natl Acad Sci USA 1998; 95 (22): 13176–81.
Paine MF, Khalighi M, Fisher JM, et al. Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J Pharmacol Exp Ther 1997; 283 (3): 1552–62.
Hall SD, Thummel KE, Watkins PB, et al. Molecular and physical mechanisms of first-pass extraction. Drug Metab Dispos 1999; 27 (2): 161–6.
Lown KS, Ghosh M, Watkin PB. Sequences of intestinal and hepatic cytochrome P450 3A4 cDNAs are identical. Drug Metab Dispos 1998; 26 (2): 185–7.
Lown KS, Bailey DG, Fontana RJ, et al. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J Clin Invest 1997; 99 (10): 2545–53.
Thummel KE, O’shea D, Paine MF, et al. Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin Pharmacol Ther 1996; 59 (5): 491–502.
Lang CC, Brown RM, Kinirons MT. Decreased intestinal CYP3A4 in celiac disease: reversal after successful glutenfree diet: a potential source of interindividual variability in first-pass drug metabolism. Clin Pharmacol Ther 1996; 59 (1): 41–6.
Lechevrel M, Casson AG, Wolf CR, et al. Characterization of cytochrome P450 expression in human oesophageal mucosa. Carcinogenesis 1999; 20 (2): 243–8.
Haehner BD, Gorski JC, Vandenbranden M, et al. Bimodal distribution of renal cytochrome P450 3A activity in humans. Mol Pharmacol 1996; 50 (1): 52–9.
Sempoux C, Starkel P, Stevens M, et al. Cytochrome P450 3A proteins are expressed in B lymphocytes but not in T lymphocytes [in process citation]. Pharmacogenetics 1999; 9 (2): 263–5.
Janardan SK, Lown KS, Schmiedlin-Ren P, et al. Selective expression of CYP3 A5 and not CYP3A4 in human blood. Pharmacogenetics 1996; 6 (5): 379–85.
Stärkel P, Sempoux C, Van Den Berge V, et al. CYP 3Aproteins are expressed in human neutrophils and lymphocytes but are not induced by rifampicin. Life Sci 1999; 64 (8): 643–53.
Murray GI, Pritchard S, Melvin WT, et al. Cytochrome P450 CYP3A5 in the human anterior pituitary gland. FEBS Lett 1995; 364 (1): 79–82.
Ladona MG, Spalding DJ, Ekman L, et al. Human fetal and adult liver metabolism of ethylmorphine. Relation to immunodetected cytochrome P-450 PCN and interactions with important fetal corticosteroids. Biochem Pharmacol 1989; 38 (19): 3147–55.
Tanaka E. Clinically important pharmacokinetic drug-drug interactions: role of cytochrome P450 enzymes. J Clin Pharmacol Ther 1998; 23: 403–16.
Li AP, Reith MK, Rasmussen A, et al. Primary human hepatocytes as a tool for the evaluation of structure-activity relationship in cytochrome P450 induction potential of xenobiotics: evaluation of rifampin, rifapentine and rifabutin. Chem Biol Interact 1997; 107 (1–2): 17–30.
Vital Durand D, Hampden C, Boobis AR, et al. Induction of mixed function oxidase activity in man by rifapentine (MDL 473), a long-acting rifamycin derivative. Br J Clin Pharmacol 1986; 21 (1): 1–7.
Backman JT, Kivisto KT, Olkkola KT, et al. The area under the plasma concentration-time curve for oral midazolam is 400-fold larger during treatment with itraconazole than with rifampicin. Eur J Clin Pharmacol 1998; 54: 53–8.
Kovacs SJ, Martin DE, Everitt DE, et al. Urinary excretion of 6 beta-hydroxycortisol as an in vivo marker for CYP3A induction: applications and recommendations. Clin Pharmacol Ther 1998; 63 (6): 617–22.
Kerr BM, Thummel KE, Wurden CJ, et al. Human liver carbamazepine metabolism. Role of CYP3A4 and CYP2C8 in 10,11-epoxide formation. Biochem Pharmacol 1994; 47 (11): 1969–79.
Jones TE. The use of other drugs to allow a lower dosage of cyclosporin to be used. Therapeutic and pharmacoeconomic considerations. Clin Pharmacokinet 1997; 32 (5): 357–67.
Lucey MR, Kolars JC, Merion RM, et al. Cyclosporin toxicity at therapeutic blood levels and cytochrome P-450 IIIA. Lancet 1990; 335 (8680): 11–5.
Lehmann JM, AMcKee DD, Watson MA, et al. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest 1998; 102 (5): 1016–23.
Ouellet D, Hsu A, Granneman GR, et al. Pharmacokinetic interaction between ritonavir and clarithromycin. Clin Pharmacol Ther 1998; 64: 355–62.
Maurice M, Pichard L, Daujat M, et al. Effects of imidazole derivatives on cytochromes P450 from human hepatocytes in primary culture. Faseb J 1992; 6 (2): 752–8.
Varis T, Kaukonen K-M, Kivisto T, et al. Plasma concentrations and effects of oral methylprednisolone are considerably increased by itraconazole. Clin Pharmacol Ther 1998; 64: 363–8.
Ducharme MP, Warbasse LH, Ewdards DJ. Disposition of intravenous and oral cyclosporine after administration with grapefruit juice. Clin Pharmacol Ther 1995; 57 (5): 485–91.
Rau SE, Bend JR, Arnold JMO, et al. Grapefruit juice-terfenadine single-dose interaction: magnitude, mechanism and relevance. Clin Pharmacol Ther 1996; 61 (4): 401–9.
Schuetz JD, Schuetz EG, Thottassery JV, et al. Identification of a novel dexamethasone responsive enhancer in the human CYP3A5 gene and its activation in human and rat liver cells. Mol Pharmacol 1996; 49 (1): 63–72.
Gibbs MA, Thummel KE, Shen DD, et al. Inhibition of cytochrome P-450 3A (CYP3A) in human intestinal and liver microsomes: comparison of Ki values and impact of CYP3A5 expression. Drug Metab Dispos 1999; 27 (2): 180–7.
Greuet J, Pichard L, Bonfils C, et al. The fetal specific gene CYP3A7 is inducible by rifampicin in adult human hepatocytes in primary culture. Biochem Biophys Res Commun 1996; 225 (2): 689–94.
Hakkola J, Tanaka E, Pelkonen O. Developmental expression of cytochrome P450 enzymes in human liver. Pharmacol Toxicol 1998; 82 (5): 209–17.
Cresteil T, Beaune P, Kremers P, et al. Immunoquantification of epoxide hydrolase and cytochrome P-450 isozymes in fetal and adult human liver microsomes. Eur JBiochem 1985; 151 (2): 345–50.
Treluyer JM, cheron G, Sonnier M, et al. Cytochrome P-450 expression in sudden infant death syndrome. Biochem Pharmacol 1996; 52 (3): 497–504.
Shimada T, Yamazaki H, Mimura M, et al. Characterization of microsomal cytochrome P450 enzymes involved in the oxidation of xenobiotic chemicals in human fetal liver and adult lungs. Drug Metab Dispos 1996; 24 (5): 515–22.
Chiba M, Nishime JA, Lin JH, et al. In vitro metabolism of indinavir in the human fetal liver microsomes. Drug Metab Dispos 1997; 25 (10): 1219–22.
Hakkola J, Pasanen M, Purkunen R, et al. Expression of xenobiotic-metabolizing cytochrome P450 forms in human adult and fetal liver. Biochem Pharmacol 1994; 48 (1): 59–64.
Schuetz JD, Kauma S, Guzelaian PS. Identification of the fetal liver cytochrome CYP3A7 in human endometrium and placenta. J Clin Invest 1993; 92 (2): 1018–24.
Piafsky KM, Rane A. Formation of carbamazepine epoxide in human fetal liver. Drug Metab Dispos 1978; 6 (4): 502–3.
Jacqz-Aigrain E, Funck-Brentano C, Cresteil T. CYP2D6- and CYP3A-dependent metabolism of dextromethorphan in humans. Pharmacogenetics 1993; 3: 197–204.
Treluyer JM, Jacqz-Aigrain E, Alvarez F, et al. Expression of CYP2D6 in developing human liver. Eur J Biochem 1991; 202 (2): 583–8.
Treluyer JM, Gueret G, Cheron G, et al. Developmental expression of CYP2C and CYP2C-dependent activities in the human liver: in-vivo/in-vitro correlation and inducibility. Pharmacogenetics 1997; 7 (6): 441–52.
Bargetzi MJ, Toshifumi A, Gonzalez FJ, et al. Lidocaine metabolism in human liver microsomes by cytochrome P450 3A. Clin Pharmacol Ther 1989; 46 (5): 521–7.
Li Y, Yokoi T, Sasaki M, et al. Perinatal expression and inducibility of human CYP3A7 in C57BL/6N transgenic mice. Biochem Biophys Res Commun 1996; 228 (2): 312–7.
Rollins DE, von Bahr C, Glaumann H, et al. Acetaminophen: potentially toxic metabolite formed by human fetal and adult liver microsomes and isolated fetal liver cells. Science 1979; 205: 1414–6.
Maenpaa J, Pelkonen O, Cresteil T, et al. The role of cytochrome P450 3A (CYP3A) isoform(s) in oxidative metabolism of testosterone and benzphetamine in human adult and fetal liver. J Steroid Biochem Mol Biol 1993; 44 (1): 61–7.
Ohmori S, Fujiki N, Nakasa H, et al. Steroid hydroxylation by human fetal CYP3A7 and human NADPH-cytochrome P450 reductase coexpressed in insect cells using baculovirus. Res Commun Mol Pathol Pharmacol 1998; 100 (1): 15–28.
Kitada M, Kamataki T, Itahashi K, et al. Significance of cytochrome P-450 (P-450 HFLa) of human fetal livers in the steroid and drug oxidations. Biochem Pharmacol 1987; 36 (4): 453–6.
Li Y, Yokoi T, Kitamura R, et al. Establishment of transgenic mice carrying human fetus-specific CYP3A7. Arch Biochem Biophys 1996; 329 (2): 235–40.
Hashimoto H, Yanagawa Y, Sawada M, et al. Simultaneous expression of human CYP3A7 and N-acetyltransferase in Chinese hamster CHL cells results in high cytotoxicity for carcinogenic heterocyclic amines. Arch Biochem Biophys 1995; 320 (2): 323–9.
Lown K, Kolars J, Turgeon K, et al. The erythromycin breath test selectively measures P450 3A4 in patients with severe liver disease. Clin Pharmacol Ther 1992; 51 (3): 229–38.
Turgeon DK, Normolle DP, Leictman AB, et al. Erythromycin breath test predicts oral clearance of cyclosporine in kidney transplant recipients. Clin Pharmacol Ther 1992; 52 (5): 471–8.
Yeates RA, Scharpf H, Laufen H, et al. Screening for cytochrome P450 3A in man: studies with midazolam and nifedipine. J Pharm Pharmacol 1996; 48: 933–4.
Watkins B, Turgeon DK, Saenger P, et al. Comparison of urinary 6-beta-cortisol and the erythromycin breath test as measures of hepatic P450IIIA (CYP3A) activity. Clin Pharmacol Ther 1992; 52 (3): 265–73.
Hunt CM, Watkins PB, Saenger P, et al. Heterogeneity of CYP3A isoforms metabolizing erythromycin and cortisol. Clin Pharmacol Ther 1992; 51 (1): 18–23.
Sande MA, Mandell GL. Antimicrobial agents. In: Goodman Gilman A, et al., editors. The pharmacological basis of therapeutics. New York: McGraw-Hill, Inc., 1992: 1130–4.
Jacqz-Aigrain E, Daoud P, Burtin S, et al. Pharmacokinetics of midazolam during continuous infusion in critically ill neonates. Eur J Clin Pharmacol 1992; 42: 329–32.
Burtin P, Jacqz-Aigrain E, Girard P, et al. Population pharmacokinetics of midazolam in neonates. Clin Pharmacol Ther 1994; 56 (6): 615–25.
Jacqz-Aigrain E, Wood C, Robieux I. Pharmacokinetics of midazolam in critically ill neonates. Eur J Clin Pharmacol 1990; 39: 191–2.
Reves JG, Fragen RJ, Vinik HR, et al. Midazolam: pharmacology and uses. Anesthesiology 1985; 62 (3): 310–24.
Harte GJ, Gray PH, Lee TC, et al. Haemodynamic responses and population pharmacokinetics of midazolam following administration to ventilated, preterm neonates. J Paediatr Child Health 1997; 33 (4): 335–8.
Wells TG, Ellis EN, Casteel HB, et al. Pharmacokinetics of a single dosis midazolam in children [abstract]. Clin Pharmacol Ther 1991; 49: 160.
Dundee JW, Halliday NJ, Harper KW, et al. Midazolam: a review of its pharmacologic properties and therapeutic use. Drugs 1984; 28: 519–43.
Hughes J, Gills AM, Mulhearn H, et al. Steady-state plasma concentrations of midazolam in critically ill infants and children. Ann Pharmacother 1996; 30: 27–30.
Lee TC, Charles BG, Harte GJ, et al. Population pharmacokinetic modeling in very premature infants receiving midazolam during mechanism ventilation: midazolam neonatal pharmacokinetics. Anesthesiology 1999; 90 (2): 451–7.
Mathews HM, Carson IW, Lyons SM, et al. A pharmacokinetic sutyd of midazolam in paediatric patients indergoing cardiac surgery. Br J anaesth 1988; 61 (3): 302–7.
Hartwig S, Roth B, Theisohm M. Clinical experience with continuous intravenous sedation using midazolam and fentanyl in the paediatric intensive care unit. Eur J Pediatr 1991; 150: 784–8.
Walbergh EJ, Eckert J. Pharmacokinetics of intravenous (IV) and intranasal (IN) midazolam in children [abstract]. Anaesthesia 1989; 71 (3A): A1066.
Salonen M, Kanto J, Iisalo E, et al. Midazolam as an induction agent in children: a pharmacokinetic and clinical study, Anesth Analg 1987; 66 (7): 625–8 review]. Clin Chim Acta 1994; 226 (2): 171–90.
Lown KS, Kolars JC, Thummel KE, et al. Interpatient heterogeneity in expression of CYP3A4 and CYP3A5 in small bowel. Lack of prediction by the erythromycin breath test [published erratum appears in Drug Metab Dispos 1995 Mar; 23 (3): following table of contents]. Drag Metab Dispos 1994; 22 (6): 947–55.
Smith MT, Eadie MJ, O’Rouke Brophy T. The pharmacokinetics of midazolam in man. Eur J Clin Pharmacol 1981; 19: 271–8.
Payne K, Mattheyse FJ, Liebenberg D, et al. The pharmacokinetics of midazolam in paediatric patients. Eur J Clin Pharmacol 1989; 37: 267–72.
Ged C, Rouillon JM, Pichard L, et al. The increase in urinary excretion of 6 beta-hydroxycortisol as a marker of human hepatic cytochrome P450IIIA induction. Br J Clin Pharmacol 1989; 28 (4): 373–87.
Nakamura H, Hasegawa A, Kimura M, et al. Comparison of urinary 6β-hydroxycortisol/cortisol ratio between neonates and their mothers. Br J Clin Pharmacol 1999; 47: 31–4.
Nakamura H, Hirai M, Ohmori S, et al. Changes in urinary 6beta-hydroxycortisol/cortisol ratio after birth in human neonates. Eur J Clin Pharmacol 1998; 53 (5): 343–6.
Vauzelle-Kervroedan F, Rey E, Pariente-Khayat A, et al. Non invasive in vivo study of the maturation of CYP IIIA in neonates and infants. Eur J Clin Pharmacol 1996; 51 (1): 69–72.
Johnson CE, Beekman RH, Kostyshak DA, et al. Pharmacokinetics and pharmacodynamics of nifedipine in children with bronchopulmonary dysplasia and pulmonary hypertension. Pediatr Res 1991; 29 (5): 500–3.
Tanaka E. Clinical importance of non-genetic and genetic cytochrome P-450 function tests in liver disease. J Clin Pharmacol Ther 1998; 23: 161–70.
Cooney GF, Habucky K, Hoppu K. Cyclosporin pharmacokinetics in paediatric transplant recipients. Clin Pharmacokinet 1997; 32 (6): 481–95.
Masri MA, Dhawan VS, Hayes K, et al. Cyclosporine dosage according to pharmacokinetic profiles leads to better graft and patient survival rates and a decrease in cyclosporine consumption. Transplant Proc 1992; 24 (5): 1718–20.
Lindholm A, Welsh M, Rutzky L, et al. The adverse impact of high cyclosporine clearance rates on the incidences of acute rejection and graft loss. Transplantation 1993; 55 (5): 985–93.
Hoppu K, Koskimies O, Holmberg C, et al. Evidence for prehepatic metabolism of oral cyclosporine in children. Br J Clin Pharmacol 1991; 32 (4): 477–81.
Lown KS, Mayo RR, Leichtman AB, et al. Role of intestinal P-glycoprotein (mdrl) in interpatient variation in the oral bioavailability of cyclosporine. Clin Pharmacol Ther 1997; 62 (3): 248–60.
Schwartz M, Holst B, Facklam D, et al. FK 506 in liver transplantation: correlation of whole blood levels with efficacy and toxicity. The US Multicenter FK 506 Dose Optimization. Transplant Proc 1995; 27(1): 1107.
Yasuhara M, Hashida T, Toraguchi M, et al. Pharmacokinetics and pharmacodynamics of FK 506 in pediatric patients receiving living-related donor liver transplantations. Transplant Proc 1995; 27 (1): 1108–10.
Filler G, Grygas E, Mai I, et al. Pharmacokinetics of tacrolimus (FK 506) in children and adolescents with renal transplants. Nephrol Dial Transplant 1997; 12 (8): 1668–71.
Sonnichsen DS, Ribeiro RC, Luo X, et al. Pharmacokinetics and pharmacodynamics of 21-day continuous oral etoposide in pediatric patients with solid tumors. Clin Pharmacol Ther 1995; 58 (1): 99–107.
Boos J, Krumpelmann S, Schulze-Westhoff P, et al. Steady-state levels and bone marrow toxicity of etoposide in children and infants: does etoposide require age-dependent dose calculation? J Clin Oncol 1995; 13 (12): 2954–60.
Pynnönen S, Kanot J, Sillanpää M, et al. Carbamazepine: placental transport, tissue concentrations in foetus and newborn, and level in milk. Acta Pharmacol Toxicol (Copenh) 1977; 41 (3): 244–53.
Suzuki Y, Cox S, Hayes J, et al. Carbamazepine age-dose ratio relationship in children. Ther Drug Monit 1991; 13 (3): 201–8.
Korinthenberg R, Haug C, Hannak D. The metabolization of carbamazepine to CBZ-10,11-epoxide in children from the newborn age to adolescence. Neuropediatrics 1994; 25 (4): 214–6.
Wiley JFD, Gelber ML, Henretig FM, et al. Cardiotoxic effects of astemizole overdose in children. J Pediatr 1992; 120 (5): 799–802.
Tobin JR, Doyle TP, Ackerman AD, et al. Astemizole-induced cardiac conduction disturbances in a child. JAMA 1991; 266 (19): 2737–40.
Hoppu K, Tikanoja T, Tapanainen P, et al. Accidental astemizole overdose in young children. Lancet 1991; 338 (8766): 538–40.
Bernardini S, Semama DS, Huet F, et al. Effects of cisapride on QTc interval in neonates. Arch Dis Child Fetal Neonatal Ed 1997; 77 (3): F241–3.
Bedu A, Lupoglaoff JM, Faure C, et al. Cisapride high dosage and long QT interval [letter; comment]. J Pediatr 1997; 130 (1): 164.
Charles B, Preechagoon Y, Donovan T. Population pharmacokinetics of cisapride in young infants with gastro-esophagal reflux. Ther Drug Monit 1997; 19 (5): 596.
Cazeneuve C, Pons G, Rey E, et al. Biotransformation of caffeine in human liver microsomes from foetuses, neonates, infants and adults. Br J Clin Pharmacol 1994; 377: 405–12.
Besunder JB, Reef MD, Blumer JL. Principles of drug biodisposition in the neonate. Acritical evaluation of the pharmacokinetic-pharmacodynamic interface (Pt I and Pt II). Clin Pharmacokinet 1988; 14: 189–216, 261–86.
Sonnier M, Cresteil T. Delayed ontogenesis of CYP1A2 in the human liver. Eur J Biochem 1998; 251 (3): 893–8.
Sereni F, Mandelli M, Principi N, et al. Induction of drag metabolizing enzyme activities in the human fetus and in the newborn infant. Enzyme 1973; 15 (1): 318–29.
Hiller A, Olkkola KT, Isohanni P, et al. Unconsciousness associated with midazolam and erythromycin [see comments]. Br J Anaesth 1990; 65 (6): 826–8.
Kawashiro T, Yamashita K, Zhao XJ, et al. A study on the metabolism of etoposide and possible interactions with antitumor or supporting agents by human liver microsomes. J Pharmacol Exp Ther 1998; 286 (3): 1294–300.
Bisogno G, Cowie F, Boddy A, et al. High-dose cyclosporin with etoposide — toxicity and pharmacokinetic interaction in children with solid tumours. Br J Cancer 1998; 77 (12): 2304–9.
Rane A, Bertilsson L, Palmer L. Disposition of placentally transferred carbamazepine (Tegretol) in the newborn. Eur J Clin Pharmacol 1975; 8 (3–4): 283–4.
Riva R, Albani F, Contin M, et al. Pharmacokinetic interactions between antiepileptic drags. Clin Pharmacokinet 1996; 31 (6): 470–93.
Liu H, Delgado MR. Interactions of phenobarbital and phenytoin with carbamazepine and its metabolites’ concentrations, concentration ratios, and level/dose ratios in epileptic children. Epilepsia 1995; 36 (3): 249–54.
Jounaidi Y, Guzelian PS, Maurel P, et al. Sequence of the 5′-flanking region of CYP3A5: comparative analysis with CYP3A4 and CYP3A7. Biochem Biophys Res Commun 1994; 205 (3): 1741–7.
Liddle C, Goodwin BJ, George J, et al. Separate and interactive regulation of cytochrome P450 3 A4 by triiodothyronine, dexamethasone, and growth hormone in cultured hepatocytes. J Clin Endocrinol Metab 1998; 83 (7): 2411–6.
Murray DJ, Crom WR, Reddick WE, et al. Liver volume as a determinant of drug clearance in children and adolescents. Drug Metab Dispos 1995; 23 (10): 1110–6.
Relling MV, Harrison PL, Evan WE. Cytochrome P450 in normal pediatric vs. adult human liver. Clin Pharmacol Ther 1999; 65 (2): 139A.
Holford NH. A size standard for pharmacokinetics. Clin Pharmacokinet 1996; 30 (5): 329–32.
Abdel-Razzak Z, Loyer P, Fautrel A, et al. Cytokines down-regulate expression of major cytochrome P-450 enzymes in adult human hepatocytes in primary culture. Mol Pharmacol 1993; 44 (4): 707–15.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Wildt, S.N., Kearns, G.L., Leeder, J.S. et al. Cytochrome P450 3A. Clin Pharmacokinet 37, 485–505 (1999). https://doi.org/10.2165/00003088-199937060-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-199937060-00004