Abstract
The 3-hydroxy-3-methyl coenzyme A (HMG-CoA) reductase inhibitors or statins, specifically inhibit the enzyme HMG-CoA in the liver, thereby inhibiting the rate limiting step in cholesterol biosynthesis and so reducing plasma cholesterol levels. Numerous studies have consistently demonstrated that cholesterol lowering with statin therapy reduces morbidity and mortality from coronary heart disease, whilst recent evidence has demonstrated that benefits of statin therapy may also extend into stroke prevention.
Since hypercholesterolaemia is a chronic condition, the long-term safety and tolerability of these agents is an important issue. Numerous large-scale clinical trials have consistently demonstrated a positive safety and tolerability profile for statins. Hepatic, renal and muscular systems are rarely affected during statin therapy, with adverse reactions involving skeletal muscle being the most common, ranging from mild myopathy to myositis and occasionally to rhabdomyolysis and death. Postmarketing data supports the positive safety and tolerability profile of statins, with an overall adverse event frequency of less than 0.5% and a myotoxicity event rate of less than 0.1%.
The recent withdrawal of cerivastatin from the world market due to deaths from rhabdomyolysis has, however, focused attention on the risk of adverse events and in particular myotoxicity associated with statins. Indeed, initial clinical trial data supports postmarketing data, demonstrating a higher incidence of myotoxicity associated with cerivastatin, particularly when used in combination with fibrates.
The potential mechanisms underlying statin-induced myotoxicity are complex with no clear consensus of opinion. Candidate mechanisms include intracellular depletion of essential metabolites and destabilisation of cell membranes, resulting in increased cytotoxicity.
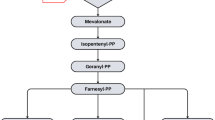
Cytochrome P450 3A4 is the main isoenzyme involved in statin metabolism. Reduced activity of this enzyme due to either reduced expression or inhibition by other drugs prescribed concomitantly such as cyclosporin or itraconazole may increase drug bioavailability and the risk of myotoxicity. Such factors may partly account for the interindividual variability in susceptibility to statin-induced myotoxicity, although other as of yet unclarified, genetic factors may also be involved.
The risk of rhabdomyolysis is increased with combination fibrate-statin therapy, with initial evidence suggesting that gemfibrozil-statin combination may particularly increase the risk of myotoxicity, with pharmacodynamic as well as pharmacokinetic mechanisms being involved.





Similar content being viewed by others
References
Menotti A, Blackburn H, Kromhout D, et al. Changes in population cholesterol levels and coronary heart disease deaths in seven countries. Eur Heart J 1997; 18(4): 566–71
Kannel WB, Larson M. Long-term epidemiologic prediction of coronary disease: the Framingham experience. Cardiology 1993; 82(2-3): 137–52
Stamler J, Daviglus ML, Garside DB, et al. Relationship between baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular and all cause mortality and longevity. JAMA 2000; 284(3): 311–8
Vogel RA. Coronary risk factors, endothelial function and atherosclerosis: a review. Clin Cardiol 1997; 54(1): 1–8
Ross R. The pathogenesis of atehrosclerosis: a perspective for the 1990s. Nature 1993; 362: 801–9
Scandanavian Simvastatin Survival group. Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandanavian Simvastatin Survival Study (4S). Lancet 1994; 344: 1383–9
Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolaemia. West of Scotland coronary prevention study. N Engl J Med 1995; 333(20): 1301–7
Nawrocki JW, Weiss SR, Davidson MH, et al. Reduction of LDL cholesterol by 25-60% in patients with primary hypercholsterolaemia by atorvastatin, a new HMG-CoA reductase inhibitor. Arterioscler Thromb Vasc Biol 1995; 15(5): 678–82
The Pravastatin Multinational Study Group for Cardiac Risk Patients. Effects of pravastatin on in patients with serum cholesterol levels between 5.2 to 7.8 mmol/l plus two additional atherosclerotic risk factors. The pravastatin multinational study group for cardiac risk patients. Am J Cardiol 1993; 72 (14): 1031–7
Bradford RH, Shear CL, Chremos AN, et al. Expanded clinical evaluation of lovastati (EXCEL) study results: efficacy in modifying plasma lipoproteins and adverse event profile in 8245 patients with moderate hypercholesterolaemia. Arch Intern Med 1991; 151(1): 43–9
Stein E, Sprecher D, Allenby KS, et al. Cerivastatin a new potent synthetic HMG Co A reductase inhibitor: effect of 0.2mg daily in subjects with hypercholesterolaemia. European Study Group. Cardiovasc Pharmacol 1997; 2(1): 7–16
Herbert PR, Gaziano JM, Chan KS, et al. Cholesterol lowering with statin drugs, risk of stroke and total mortality: an overview of randomized trials. JAMA 1997; 278(4): 1301–7
Alberts AW, Chen J, Kuron G, et al. Mevinolin: a highly potent competitive inhibitor of hydroxymetylglutaryl-co enzyme A reductase and a cholesterol lowering agent. Biochemistry 1980; 77(7): 3957–61
Farmer JA, Torre-Amione G. Comparative tolerability of the HMG-CoAreductase inhibitors. Drug Saf 2000; 23(3): 197–213
Ucar M, Mjorndal T, Dahlqvist R, et al. HMG-CoA reductase inhibitors and myotoxicity. Drug Saf. 2000; 22(6): 441–57
Gaist D, Jeppesen D, Andersen M, et al. Statins and the risk of polyneuropathy: a case control study. Neurology 2002; 58(9): 1333–7
Pentikanen PJ, Saraheimo M, Schwartz JI, et al. Comparative pharmacokinetics of lovastatin, simvastatin and pravastatin in human. J Clin Pharmacol 1992; 32: 136–40
Desager J-P, Horsmans Y. Clinical pharamacokinetics of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors. Clin Pharmacokinet 1996; 31(6): 348–71
Lea AP, McTavish D. Atorvastatin a review of its pharamacology and therapeutic potential in the management of hyperlipidaemia. Drugs 1997; 53(3): 469–73
Plosker GL, Dunn CJ, Figgitt DP. Cerivastatin: a review of its pharamacological properties and therapeutic efficacy in the management of hypercholesterolaemia. Drugs 2000; 60(5): 1179–206
Christians U, Jacobsen W, Floren LC. Metabolism and drug interactions of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors: are the statins mechanistically similar? Pharmacol Ther 1998; 80(1): 1–34
Davidson MH. Does differing metabolism by cytochrome P450 have clinical importance? Curr Atheroscler Rep 2000; 2: 14–9
Prueksaritanont T, Gorham LM, Ma B, et al. In-vitro metabolism of simvastatin in humans identification of metabolizing enzyme systems and effect of the drug on hepatic P450s. Drug Metab Dispos 1997; 25(10): 2397–402
Zhou LX, Finley DK, Hassell AE, et al. Pharmacokinetic interaction between isradipine and lovastatin in normal, femaleand male volunteers. J Pharmacol Exp Ther 1995; 273(1): 121–7
Chong PH, Seeger JD. Atorvastatin calcium: an addition to HMG-CoA reductase inhibitors. Pharmacotherapy 1997; 17(6): 1157–77
Wolfgang M. Rational assessment of the interaction profile of cerivastatin supports its low propensity for drug interaction. Drugs 1998; 56Suppl. 1: 15–23
Haria M, McTavish D. Pravastatin: a reappraisal of its pharmacological properties and clinical effectiveness in the management of coronary heart disease. Drugs 1997; 53(2): 299–336
Muck W, Ochmann K, Rhode G, et al. Influence of erythromycin pre- and co-treatment on single dose pharmacokinetics of the HMG-CoA reductase inhibitor cerivastatin. Eur J Clin Pharmacol 1998; 53: 469–73
Pichard L, Domergue J, Fourtanier G, et al. Metabolism of the new immumosuppresor cyclosporin G by human liver cytochrome P450. Biochem Pharmacol 1996; 51(5): 591–8
Lennernas H, Fager G. Pharmacodynamics and pharmacokinetics of the HMG-CoA reductase inhibitors: similarities and differences. Clin Pharmacokinet 1997; 32: 403–25
Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial study group. 1999. N Engl J Med 1999; 341: 410–8
Shek A, Ferrill MJ. Statin-fibrate combination therapy. Ann Pharmacother 2001; 35(7-8): 908–17
Backman JT, Kyrklund C, Kivisto KT, et al. Plasma concentrations of active simvastatin acid are increased by gemfibrozil. Clin Pharmacol Ther 2000; 68: 122–9
Adkins JC, Faulds D. Micronised fenofibrate: a review of its pharmacodynamic properties and clinical efficacy in the management of dyslipidaemia. Drugs 1997; 54(4): 615–33
Schoonjans K, Steals B, Auwerx J. Role of peroxisome proliferator activated receptor in mediating effects of fibrates and fatty acids on gene expression. J Lipid Res 1996; 37: 907–25
Mu YM, Yanase T, Nishi Y, et al. Combined treatment with specific ligands for PPARgamma: RXR nuclear receptor system markedly inhibits the expression of cytochrome P450arom in human granulosa cancer cells. Mol Cell Endocrinol 2001; 181(1-2): 239–48
Palmer CN, Hsu MH, Muerhoff AS, et al. Interaction of the peroxisome proliferator-activated receptor alpha with the retinoid X receptor alpha unmasks a cryptic peroxisome proliferator response element that overlaps an ARP-1-binding site in the CYP4A6 promoter. J Biol Chem 1994; 269(27): 18083–9
Olefsky JM, Saltiel AR. PPAR gamma and the treatment of insulin resistance. Trends Endocrinol Metab 2000; 9: 362–8
Kreider M, Cohen B, Freed M, et al. Rosiglitazone in combination with a statin: effect on lipid profile in patients with type 2 diabetes. The Endocrine Society’s 83rd Annual Meeting; 2001 Jun 20-23; Denver (CO): 3–55
Lane RJM, Mastaglia FL. Drug-induced myopathies in man. Lancet 1978; II(8089): 562–6
Fichtl B, Kurz H. Binding of drugs to human muscle. Eur J Clin Pharmacol 1978; 14: 335–40
Lofberg M, Jankala H, Paetau A, et al. Metabolic causes of recurrent rhabdomyolysis. Acta Neurol Scand 1998; 98(4): 268–75
Kakko JP. Rhabdomyolysis. In: Goldman L, Bennett JC, editors. Cecil textbook of medicine. Philadelphia (PA); WB Saunders, 2001: 522–5
Hino I, Akama H, Furuya T, et al. Prava-induced rhabdomyolysis in a patient with mixed connective tissue disease. Arthritis Rheum 1996; 39(7): 1259–60
Bradford RH, Shear CL, Chremos AN, et al. Expanded Clinical Evaluation of Lovastatin (EXCEL) study results: two-year efficacy and safety follow-up. Am J Cardiol 1994 1; 74(7): 667–73
Pfeffer MA, Sacks FM, Moye LA, et al. Influence of baseline lipids on effectiveness of pravastatin in the CARE Trial. Cholesterol And Recurrent Events. J Am Coll Cardiol 1999; 33(1): 125–30
WOSCOPS. Influence of pravastatin and plasma lipids on clinical events in theWest of ScotlandCoronary Prevention Study (WOSCOPS). Circulation 1998 Apr 21; 97 (15): 1440–5
Heart Protection Study Group. MRC/BHF heart protection study of cholesterol lowering and antioxidant vitamin supplementation in a wide range of patients at increased risk of coronary heart disease. Eur Heart J 1999; 20 (10): 724–41
Downs JR, Clearfield M, Tyroler HA, et al. Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TEXCAPS): additional perspectives on tolerability of long-term treatment with lovastatin. Am J Cardiol 2001 1; 87(9): 1074–9
Long-term effectiveness and safety of pravastatin in 9014 patients with coronary heart disease and average cholesterol concentrations: the LIPID (Long-Term Intervention with Pravastatin in Ischaemic Disease) Study group. Lancet 2002 Apr 20; 359 (9315): 1379–87
Pfeffer MA, Keech A, Sacks FM, et al. Safety and tolerability of pravastatin in long-term clinical trials: pravastatin pooling project [abstract]. Eur Heart J 2001; 22: 271
Herd JA, Ballantyne CM, Farmer JA, et al. Effects of fluvastatin on coronary atherosclerosis in patients with mild to moderate cholesterol elevations. Lipoprotein and Coronary Atherosclerosis Study [LCAS]. Am J Cardiol 1997; 80: 278–86
Waters D. Is a mechanical or a metabolic approach superior in the treatment of coronary disease? Results of the atorvastatin versus revascularization (AVERT) trial. Eur Heart J 2000; 21(13): 1029–31
Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischaemic events in acute coronary syndromes: The MIRACL study: a randomized controlled trial. JAMA 2001; 285(13): 1711–8
Malhotra HS, Goa KL. Atorvastatin: an updated review of its pharmacological properties and use in dyslipidaemia. Drugs 2001; 61(12): 1835–81
Jacobson TA, Amorosa LF. Combination therapy with fluvastatin and niacin in hypercholesterolaemia: a preliminary report on safety. Am J Cardiol 1994; 73(14): 25D–9D
Stein E. Cerivastatin and primary hyperlipidaemia: a multicenter analysis of safety and efficacy. Am J Cardiol 1998; 82: 40J–6J
Muck W. Clinical pharmacokinetics of cerivastatin. Clin Pharmacokinet 2000; 39(2): 99–116
Ose L, Luurila O, Eriksson J, et al. Cerivastatin gender effect: sub-analyses of results from a multinational, randomised, double-blind study. Cerivastatin Study Group. Curr Med Res Opin 2000; 16: 80–7
Farmer JA. Learning from the cerivastatin experience. Lancet 2001 27; 358(9291): 1383–5
Roca B, Calvo B, Monferrer R. Severe rhabdomyolysis and cerivastatin-gemfibrozil combination therapy. Ann Pharmacother 2002;36(4): 730–1
Feeney ER, Calissi PT, Pylypchuk GB. Rhabdomyolysis from high-dose cerivastatin therapy [abstract]. Ann Pharmacother 2002; 36(6): 1106
Kogan AD, Orensterin S. Lova-induced acute rhabdomyolysis. Postgrad Med J 1990; 66: 293–6
Langer T, Levy RI. Acute muscular syndrome associated with administration of clofibrate. N Engl J Med 1968; 279(16): 856–8
Gorriz JL, Sancho A, Lopez-Martin JM, et al. Rhabdomyolysis and acute renal failure associated with gemfibrozil therapy. Nephron 1996; 74(2): 437–8
Goldman JA, Fishman AB, Lee JE, et al. The role of cholesterol lowering agents in drug induced rhabdomyolysis and polymyositis. Arthritis Rheum 1989; 32: 358–9
Sonoda Y, Gotow T, Kuriyama M, et al. Electrical myotonia of rabbit skeletal muscles by HMG-CoA reductase inhibitors. Muscle Nerve 1994; 17(8): 891–7
Gadbut AP, Caruso AP, Gabler JB. Differential sensitivity of C2-C12 striated muscle cells to lovastatin and pravastatin. J Mol Cell Cardiol 1995; 27(10): 1191–9
Hochgraf E, Levy Y, Aviram M, et al. Lovastatin decreases plasma and platelet cholesterol levels and normalizes elevated platelet fluidity and aggregation in hypercholesterolaemic patients. Metabolism 1994; 43(1): 59–64
Levy J, Leibowitz R, Aviram M, et al. Reduction of plasma cholesterol by lovastatin in patients with severe hypercholesterolaemia. Br J Clin Pharmacol 1992; 34(5): 427–30
Morita I, Sato I, Ma L, et al. Enhancement of membrane fluidity in cholesterol-poor endothelial cells pre-treated with simvastatin. Endothelium 1997; 5(2): 107–13
Lijnen P, Celis H, Fagard R, et al. Influence of cholesterol lowering on plasma membrane lipids and cationic transport systems. J Hypertens 1994; 12(1): 59–64
Bank WJ, Dimauro S, Bonilla E, et al. A disorder of muscle lipid metabolism and myoglobinuria: absence of carnitine palmityl transferase. N Engl J Med 1975; 292: 443–9
Chu PH, Chen WJ, Chiang CW, et al. Rhabdomyolysis, acute renal failure and hepatopathy induced by lovostatin monotherapy. Jpn Heart J 1997; 38(4): 541–5
Gebhard RL, Ewing SL, Schlasner LA, et al. Effect of 3-hydroxy-3-methyl coenzyme Areductase inhibiton on human gut mucosa. Lipids 1991; 26(7): 492–4
Di Mauro S, Bonilla E, Davidson M, et al. Mitochondria in neuromuscular disorders. Biochem Biophys Acta 1998; 1366(1-2): 199–210
Laaksonen R, Jokelainen K, Sahi T, et al. Decreases in serum ubiquinone concentrations do not result in reduced levels in muscle tissue during short-term simvastatin treatment in humans. Clin Pharmacol Ther 1995; 57(1): 62–6
Giordano N, Senesi M, Mattii G, et al. Polymyositis associated with simvastatin. Lancet 1997; 349: 1600–1
Schalke BB, Schmitdt B, Toyka K, et al. Pravastatin associated inflammatory myopathy. N Engl J Med 1992; 327(9): 649–50
Kantola T, Kivisto KT, Neuvonen PJ, et al. Erythromycin and verapamil considerably increase serum simvastatin and simvastatin acid concentrations. Clin Pharmacol Ther 1998; 64(2): 177–82
Shimada T, Yamazaki H, Mimura M, et al. Interindividual variations in human liver cytochrome P450 enzymes involved in the oxidation of drugs, carcinogens, and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther 1994; 270(1): 414–23
Davidson MH, Stein EA, Dujovne CA, et al. The efficacy and six-week tolerability of simvastatin 80 and 160 mg/day. Am J Cardiol 1997; 257(79): 38–42
Masters BA, Palmoski MJ, Flint OP, et al. In vitro myotoxicity of the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, pravastatin, lovastatin and simvastatin, using neonatal rat skeletal myocytes. Toxicol Appl Pharmacol 1995; 131(1): 163–74
Duell PB, Connor WE, Illingworth DR. Rhabdomyolysis after atorvastatin with gemfibrozil. Am J Cardiol 1998; 81(3): 368–9
Van Puijenbroek EP, Du Buf-Vereijken PW, Spooren PF, et al. Possible increased risk of rhabdomyolysis during concomitant use of simvastatin and gemfibrozil. J Intern Med 1996; 240(6): 403–4
Backman JT, Kyrklund C, Kivisto KT, et al. Plasma concentrations of active simvastatin acid are increased by gemfibrozil. Clin Pharmacol Ther 2000; 68(2): 122–9
Kyrklund C, Backman JT, Kivisto KT, et al. Plasma concentrations of lovastatin are increased by gemfibrozil but not by bezafibrate. Clin Pharmaco Ther 2001; 69(5): 340–5
Wen X, Wang JS, Backman JT, et al. Gemfibrozil is a potent inhibitor of human cytochrome P450 2C9. Drug Metab Dispos 2001; 29(11): 1359–61
Shek A, Ferrill MJ. Statin-fibrate combination therapy. Ann Pharmacother 2001; 35: 908–17
Acknowledgements
Postmarketing statin prescription data for the UK were provided courtesy of Merck Sharp & Dohme using UK Mediplus data from IMS HEALTH, February 2002 update.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Evans, M., Rees, A. Effects of HMG-CoA Reductase Inhibitors on Skeletal Muscle. Drug-Safety 25, 649–663 (2002). https://doi.org/10.2165/00002018-200225090-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200225090-00004