Abstract
Two intramolecularly quenched fluorogenic peptides containing o-aminobenzoyl (Abz) and ethylenediamine 2,4-dinitrophenyl (EDDnp) groups at amino- and carboxyl-terminal amino acid residues, Abz-<!-- $MVD$:face("Times") -->DArg-Arg-Leu-EDDnp (Abz-<!-- $MVD$:face("Times") -->DRRL-EDDnp) and Abz-<!-- $MVD$:face("Times") -->DArg-Arg-Phe-EDDnp (Abz-<!-- $MVD$:face("Times") -->DRRF-EDDnp), were selectively hydrolyzed by neutral endopeptidase (NEP, enkephalinase, neprilysin, EC 3.4.24.11) at the Arg-Leu and Arg-Phe bonds, respectively. The kinetic parameters for the NEP-catalyzed hydrolysis of Abz-<!-- $MVD$:face("Times") -->DRRL-EDDnp and Abz-<!-- $MVD$:face("Times") -->DRRF-EDDnp were Km = 2.8 µM, kcat = 5.3 min-1, kcat/Km = 2 min-1 µM-1 and Km = 5.0 µM, kcat = 7.0 min-1, kcat/Km = 1.4 min-1 µM-1, respectively. The high specificity of these substrates was demonstrated by their resistance to hydrolysis by metalloproteases [thermolysin (EC 3.4.24.2), angiotensin-converting enzyme (ACE; EC 3.4.24.15)], serineproteases [trypsin (EC 3.4.21.4), <!-- $MVD$:face("Symbol") --><FONT FACE="Symbol">a</font>-chymotrypsin (EC 3.4.21.1)] and proteases present in tissue homogenates from kidney, lung, brain and testis. The blocked amino- and carboxyl-terminal amino acids protected these substrates against the action of aminopeptidases, carboxypeptidases and ACE. Furthermore, <!-- $MVD$:face("Times") -->DR amino acids ensured total protection of Abz-<!-- $MVD$:face("Times") -->DRRL-EDDnp and Abz-<!-- $MVD$:face("Times") -->DRRF-EDDnp against the action of thermolysin and trypsin. Leu-EDDnp and Phe-EDDnp were resistant to hydrolysis by <!-- $MVD$:face("Symbol") --><FONT FACE="Symbol">a</font>-chymotrypsin. The high specifity of these substrates suggests their use for specific NEP assays in crude enzyme preparations
neutral endopeptidase; enkephalinase; neprilysin; fluorogenic substrates; phosphoramidon
Braz J Med Biol Res, October 1997, Volume 30(10) 1157-1162 (Short Communication)
1Laboratório de Neurobiologia Molecular Humana, Departamento de Fisiologia e Farmacologia, Centro de Ciências da Saúde, Universidade Federal do Ceará, Fortaleza, CE, Brasil
2Départment de Biochimie, Faculté de Medicine, Université de Montréal, Montréal, Canada
3Departamento de Biofísica, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, SP, Brasil
 Text
Text
 References
References
 Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Correspondence and Footnotes
Abstract
Two intramolecularly quenched fluorogenic peptides containing o-aminobenzoyl (Abz) and ethylenediamine 2,4-dinitrophenyl (EDDnp) groups at amino- and carboxyl-terminal amino acid residues, Abz-DArg-Arg-Leu-EDDnp (Abz-DRRL-EDDnp) and Abz-DArg-Arg-Phe-EDDnp (Abz-DRRF-EDDnp), were selectively hydrolyzed by neutral endopeptidase (NEP, enkephalinase, neprilysin, EC 3.4.24.11) at the Arg-Leu and Arg-Phe bonds, respectively. The kinetic parameters for the NEP-catalyzed hydrolysis of Abz-DRRL-EDDnp and Abz-DRRF-EDDnp were Km = 2.8 µM, kcat = 5.3 min-1, kcat/Km = 2 min-1 µM-1 and Km = 5.0 µM, kcat = 7.0 min-1, kcat/Km = 1.4 min-1 µM-1, respectively. The high specificity of these substrates was demonstrated by their resistance to hydrolysis by metalloproteases [thermolysin (EC 3.4.24.2), angiotensin-converting enzyme (ACE; EC 3.4.24.15)], serineproteases [trypsin (EC 3.4.21.4), a-chymotrypsin (EC 3.4.21.1)] and proteases present in tissue homogenates from kidney, lung, brain and testis. The blocked amino- and carboxyl-terminal amino acids protected these substrates against the action of aminopeptidases, carboxypeptidases and ACE. Furthermore, DR amino acids ensured total protection of Abz-DRRL-EDDnp and Abz-DRRF-EDDnp against the action of thermolysin and trypsin. Leu-EDDnp and Phe-EDDnp were resistant to hydrolysis by a-chymotrypsin. The high specifity of these substrates suggests their use for specific NEP assays in crude enzyme preparations.
Key words: neutral endopeptidase, enkephalinase, neprilysin, fluorogenic substrates, phosphoramidon
Neutral endopeptidase (NEP, enkephalinase, neprilysin, EC 3.4.24.11) is a broadly specific zinc metalloendopeptidase which hydrolyzes internal peptide bonds on the amino side of hydrophobic amino acid residues in P'1 position, with Leu or Phe being the preferred amino acids. Shown to be widely distributed in various tissues, NEP is involved in the regulation and metabolism of a variety of biologically active peptides such as substance P, enkephalins, atrial natriuretic factor, bradykinin, gastrin, neurotensin, and the chemotactic peptide (1,2).
Several synthetic substrates have been developed to measure NEP activity, such as radiolabeled peptides ([3H]Leu-enkephalin, D-[3H]Ala2-Leu-enkephalin) (3,4), chromogenic peptides (glutaryl-Ala-Ala-Phe-2NA, benzyl-Gly-Arg-Arg-Leu-2NA) (5,6) and fluorogenic peptides (dansyl-D-Ala-Gly-Phe(pNO2)-Gly, dansyl-Gly-Phe(pNO2)-ßAla) (7,8).
Although the specificity of all these substrates for NEP is partial, and frequently the use of other protease inhibitors is required during incubation with NEP, they are used to monitor NEP purification, to determine its activity in different tissues under physiological and pathological conditions, as well as to compare the kinetic parameters of different forms of recombinant NEP produced by site-directed mutagenesis, to develop synthetic inhibitors which have been employed to study the physiological functions of NEP, and for clinical use through the increased level of endogenous peptides that are substrates for the enzyme (1,2,9).
Recently, we described a new intramolecularly quenched fluorogenic substrate for NEP related to Leu-enkephalin, containing o-aminobenzoyl (Abz) and ethylenediamine 2,4-dinitrophenyl (EDDnp) groups at amino- and carboxyl-terminal amino acid residues, Abz-GGDFLRRV-EDDnp (10,11). This substrate presents at least one important advantage in relation to other previously described fluorogenic substrates for NEP: its kcat/Km = 40 min-1 µM-1 is 20 times higher than that of dansyl-D-Ala-Gly-Phe(pNO2)-Gly (7) and dansyl-Gly-Phe(pNO2)-ßAla (8). Although Abz-GGDFLRRV-EDDnp also presents good specificity for NEP, since it is resistant to other metalloendopeptidases such as angiotensin-converting enzyme (ACE; EC 3.4.24.15) and thermolysin (EC 3.4.24.2), it is partially susceptible to degradation by trypsin-like enzymes which may cleave the R-R bond.
In the present study, we document two new intramolecularly quenched fluorogenic substrates for NEP, Abz-DRRL-EDDnp and Abz-DRRF-EDDnp, which were totally resistant to the action of other metalloproteases (ACE, thermolysin), serineproteases (trypsin, EC 3.4.21.4 and chymotrypsin, EC 3.4.21.1) and proteases present in homogenates of several tissues.
The internally quenched fluorogenic peptides Abz-DRRL-EDDnp and Abz-DRRF-EDDnp were synthesized by the solution method (12,13). A recombinant soluble form of NEP (rNEP) was expressed using a baculovirus/insect-cell system and purified by immunoaffinity as previously described (14,15).
A crude membrane preparation was obtained from rat tissues as described earlier (10). Briefly, rat tissues were homogenized in 8 volumes (w/v) of 50 mM Tris-HCl buffer, pH 7.5, using a Potter homogenizer, the homogenate was centrifuged for 10 min at 1,000 g and the pellet was discarded, the supernatant was centrifuged at 80,000 g for 60 min, the pellet was washed four times by resuspension in the same buffer used for homogenization and centrifuged under the conditions described above, and the resulting pellet, resuspended in 50 mM Tris-HCl buffer, pH 7.5 (1/8; w/v), was used as the enzyme source. Protein was measured by the method of Bradford (16). Angiotensin-converting enzyme was purified from guinea pig serum (17).
The synthetic substrates (Abz-DRRL-EDDnp and Abz-DRRF-EDDnp) and their enzymatic products were characterized by high-performance liquid chromatography (HPLC) as follows: the substrate (20 nmol) was incubated with purified rNEP (600 ng) or crude enzyme fractions (1-10 µl) in a final volume of 100 µl 50 mM Tris-HCl buffer, pH 7.5, at 37oC for 60 min. In inhibition assays, the enzyme was preincubated with 1 µM phosphoramidon for 20 min before incubation with substrates. The reaction was stopped by heating for 5 min at 100oC. After centrifugation at 10,000 g for 10 min, the supernatant fraction was injected into an HPLC column (Nucleosil 5 µm C18, 145 x 4.5 mm) and eluted with a 20-40% gradient of acetonitrile containing 0.05% trifluoracetic acid for a period of 50 min, at a flow rate of 1 ml/min. The intact substrate and products, detected by both UV absorbance (220 nm) and fluorescence (lem = 420 nm, lex = 320 nm) with the detectors arranged in series, were collected to identify the cleavage site by amino acid analysis (18).
The enzyme assays were monitored by measuring the fluorescence at lem = 420 nm and lex = 320 nm with a Shimadzu model F 2000 spectrofluorimeter. The 0.5 x 1 cm path-length cuvette containing 500 µl of the mixture of 50 mM Tris-HCl buffer, pH 7.5, and Abz-DRRL-EDDnp (10 µM) was placed in the thermostat cell compartment at 37oC for 5 min until temperature equilibrium of the solution was attained. When kidney homogenate (10 µl) was used, the fluorescence course was recorded continuously for 40 min. For the inhibition assay, the enzyme was preincubated with the inhibitor for 20 min at 37oC before incubation with substrates. The same conditions were used for thermolysin, chymotrypsin, trypsin and ACE at concentrations of 0.15 µg/ml, 8 µg/ml, 15 µg/ml and 10 µg/ml, respectively.
The kinetic parameters for the hydrolysis of Abz-DRRL-EDDnp and Abz-DRRF-EDDnp by rNEP were determined from a double-reciprocal Lineweaver-Burk plot. rNEP has an apparent MW of 87,000 (14) and this value was used for the calculation of kcat.
The substrate Abz-DRRL-EDDnp presented the lowest Km value (2.8 µM) similar to 3 µM obtained for the longer substrate derived from Leu-enkephalin, Abz-GGDFLRRV-EDDnp (10,11) (Table 1A). Furthermore, although Abz-DRRL-EDDnp presented a lower kcat/Km value (2 min-1 µM-1) than Abz-GGDFLRRV-EDDnp (42 min-1 µM-1) (10,11), this value was higher than those of all the short synthetic peptides presented in Table 1A.
All the substrates presented in Table 1A contain a hydrophobic residue at the P'1 position (Leu, Phe or Val), an essential condition for NEP activity (1,19). Thus, the excellent kinetic parameters of Abz-DRRL-EDDnp and Abz-GGDFLRRV-EDDnp may be also explained by interactions of their groups with subsites on the surface of NEP. In these substrates, the presence of EDDnp, a hydrophobic group at position P'2, may suggest a strong interaction with an S'2 subsite of NEP.
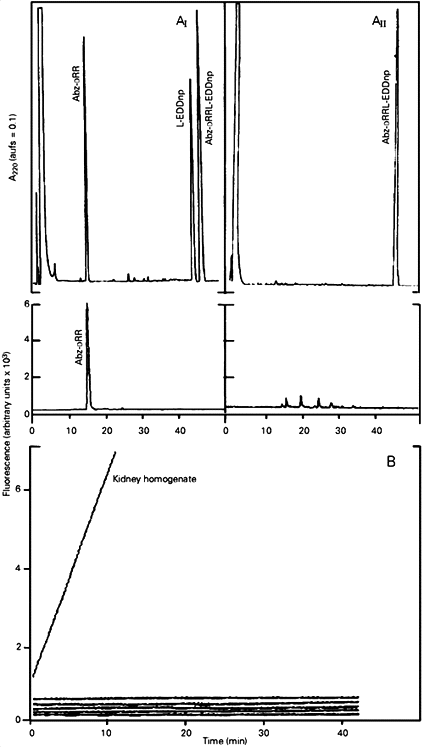
Table 1B shows the activity of NEP, other metalloendopeptidases (ACE and thermolysin) and serineproteases (trypsin and a-chymotrypsin) toward Abz-DRRL-EDDnp, Abz-DRRF-EDDnp and several other synthetic peptides. In contrast to all the other substrates, only Abz-DRRL-EDDnp and Abz-DRRF-EDDnp were totally resistant to the action of ACE, thermolysin, trypsin and a-chymotrypsin. Furthermore, the activity toward Abz-DRRL-EDDnp and Abz-DRRF-EDDnp present in kidney homogenate (Figure 1) and in tissue homogenates of lung, brain and testis (data not shown) was totally inhibited by 1 µM phosphoramidon (a highly specific NEP inhibitor), showing also the high resistance of these substrates to other tissue proteases.
- A, HPLC elution profile of Abz-DRRL-EDDnp after hydrolysis by rat kidney homogenate (AI) and inhibition of the reaction by phosphoramidon (AII). The substrate (20 nmol) was incubated with 10 µl kidney homogenate (180 µg/ml) for 60 min in 100 µl 50 mM Tris-HCl buffer, pH 7.5, at 37oC, in the presence or absence of 1 µM phosphoramidon. The reaction was stopped by heating the mixture at 100oC for 5 min followed by centrifugation at 10,000 g for 10 min. An aliquot of the supernatant (100 µl) corresponding to 90% of the reaction mixture was submitted to HPLC as described in the text. B, Continuous fluorescence recording (lem = 420 nm, lex = 320 nm) of the hydrolysis of Abz-DRRL-EDDnp (10 µM) by the enzymes presented from top to bottom: kidney homogenate (10 µl) in the absence or presence of 1 µM phosphoramidon, ACE (10 µg/ml), thermolysin (0.15 µg/ml), trypsin (15 µg/ml) and a-chymotrypsin (8 µg/ml). All incubations were performed in a final volume of 500 µl 50 mM Tris-HCl buffer, pH 7.5, at 37oC.
It has been previously demonstrated that the Abz and EDDnp groups at amino- and carboxy-terminal amino acid residues protect peptides against the action of aminopeptidases, carboxypeptidases and ACE (10,11). However, these groups do not protect peptides against the action of thermolysin or trypsin, since the substrate Abz-RRL-EDDnp, containing R at position P'2, was hydrolyzed by these enzymes (data not shown). Thus, the presence of DR at position P'2 ensured a total protection of Abz-DRRL-EDDnp and Abz-DRRF-EDDnp against thermolysin and trypsin. Finally, it may be suggested that EDDnp ensures a total protection of the L-EDDnp and F-EDDnp bonds against the action of a-chymotrypsin, since no amino acid is involved.
We have previously shown that the intramolecularly quenched fluorogenic peptide Abz-GGDFLRRV-EDDnp can be used for a rapid, highly selective and sensitive NEP assay (10,11). Indeed, since the catalytic constants obtained with Abz-GGDFLRRV-EDDnp are better than those obtained with Abz-DRRL-EDDnp, Abz-DRRF-EDDnp and other synthetic peptides shown here, it is the best substrate for use in NEP assays. As shown in Table 1B, although resistant to hydrolysis by ACE, thermolysin and a-chymotrypsin, Abz-GGDFLRRV-EDDnp was hydrolyzed at the R-R bond by trypsin, suggesting that it may also be susceptible to hydrolysis by trypsin-like enzymes present in some crude enzyme preparations. Taken together, these results show that Abz-DRRL-EDDnp and Abz-DRRF-EDDnp are substrates more specific for NEP than Abz-GGDFLRRV-EDDnp.
Finally, the results presented in this study lead us to the following conclusions: i) the previously described substrate Abz-GGDFLRRV-EDDnp (10,11), which presents the best kinetic parameters, is more suitable than Abz-DRRL-EDDnp and Abz-DRRF-EDDnp for NEP assays in purified enzyme preparations; ii) the new substrates Abz-DRRL-EDDnp and Abz-DRRF-EDDnp, which present the best specificities, are more suitable than Abz-GGDFLRRV-EDDnp for NEP assays in crude enzyme preparations.
Address for correspondence: K.M. Carvalho, Laboratório de Neurobiologia Molecular Humana, Departamento de Fisiologia e Farmacologia, CCS, UFCE, Rua Coronel Nunes de Melo, 1127, 60430-270 Fortaleza, CE, Brasil.
Research supported by CNPq, FINEP, FUNCAP, FAPESP, CAPES, and the Medical Research Council of Canada. Received April 11, 1997. Accepted August 19, 1997.
- 1. Roques BP, Noble F, Daugé V, Fournié-Zaluski MC & Beaumont A (1993). Neutral endopeptidase 24.11: structure, inhibition, and experimental and clinical pharmacology. Pharmacological Reviews, 45: 87-146.
- 2. Erdös EG & Skidgel RA (1989). Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB Journal, 3: 145-151.
- 3. Vogel Z & Altstein M (1977). The adsorption of enkephalin to porous polystyrene beads: a simple assay for enkephalin hydrolysis. FEBS Letters, 80: 332-335.
- 4. Llorens C, Malfroy B, Schwartz JC, Gacel G, Roques BP, Roy J, Morgat JL, Javoy-Agid F & Agid Y (1982). Enkephalin dipeptidyl carboxypeptidase (enkephalinase) activity: selective radioassay, properties, and regional distribution in human brain. Journal of Neurochemistry, 39: 1081-1089.
- 5. Almenoff J, Wilk S & Orlowisky M (1981). Membrane bound pituitary metalloendopeptidase: apparent identity to enkephalinase. Biochemical and Biophysical Research Communications, 102: 206-214.
- 6. Almenoff J, Wilk S & Orlowisky M (1984). Biochemical and immunological properties of a membrane-bound brain metalloendopeptidase: comparison with thermolysin-like kidney neutral metalloendopeptidase. Journal of Neurochemistry, 42: 151-157.
- 7. Florentin D, Sassi A & Roques BP (1984). A highly sensitive fluorogenic assay for "enkephalinase", a neutral metalloendopeptidase that releases tyrosin-glycine-glycine from enkephalins. Analytical Biochemistry, 141: 62-69.
- 8. Goudreau N, Guis C, Soleilhac JM & Roques BP (1994). Dns-Gly-(p-NO2)Phe-ß-Ala, a specific fluorogenic substrate for neutral endopeptidase 24.11. Analytical Biochemistry, 219: 87-95.
- 9. Gros C, Souque A, Schwartz JC, Duchier J, Cournot A, Baumer P & Lecomet JM (1989). Protection of atrial natriuretic factor against degradation, diuretic and natriuretic responses after in vivo inhibition of enkephalinase (EC 3.4.24.11) by acetorphan. Proceedings of the National Academy of Sciences, USA, 86: 7580-7584.
- 10. Carvalho KM, Boileau G, França MSF, Medeiros MAS, Camargo ACM & Juliano L (1995). A new fluorimetric assay for neutral endopeptidase (EC 3.4.24.11). Brazilian Journal of Medical and Biological Research, 28: 1055-1059.
- 11. Carvalho KM, Boileau G, Camargo ACM & Juliano L (1996). A highly selective assay for neutral endopeptidase based on the cleavage of a fluorogenic substrate related to leu-enkephalin. Analytical Biochemistry, 237: 167-173.
- 12. Chagas JR, Juliano L & Prado ES (1990). Intramolecularly quenched fluorogenic tetrapeptide substrates for tissue and plasma kallikreins. Analytical Biochemistry, 192: 419-425.
- 13. Oliveira MCF, Hirata IY, Chagas JR, Boschov P, Gomes RAS, Figueredo AFS & Juliano L (1992). Intramolecularly quenched fluorogenic peptide substrates for human renin. Analytical Biochemistry, 203: 39-46.
- 14. Fossiez F, Lemay G, Labonté N, Parmentier-Lesage F, Boileau G & Crine P (1992). Secretion of a functional form of neutral endopeptidase-24.11 from a baculovirus-infected insect cell line. Biochemical Journal, 284: 53-59.
- 15. Lemay G, Waksman G, Roques BP, Crine P & Boileau G (1989). Fusion of a cleavable signal peptide to the ectodomain of neutral endopeptidase (EC 3.4.24.11) results in the secretion of an active enzyme in COS-1 cells. Journal of Biological Chemistry, 264: 15620-15623.
- 16. Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72: 248-254.
- 17. Dorer FE, Skeggs LT, Kahn JR, Lentz KE & Levine M (1970). Angiotensin converting enzyme: method of assay and partial purification. Analytical Biochemistry, 33: 102-103.
- 18. Carvalho KM, Joudiou C, Bousseta H, Leseney AM & Cohen P (1992). A peptide-hormone-inactivating endopeptidase in Xenopus laevis skin secretion. Proceedings of the National Academy of Sciences, USA, 89: 84-88.
- 19. Spungin-Bialik A, Ben-Meir D, Fudim E, Carmeli S & Blumberg S (1996). Sensitive substrates for neprilysin (neutral endopeptidase) and thermolysin that are highly resistant to serine proteases. FEBS Letters, 380: 79-82.
- 20. Malfroy B & Schwartz JC (1982). Properties of" enkephalinase" from rat kidney: comparison of dipeptidyl-carboxypeptidase and endopeptidase activities. Biochemical and Biophysical Research Communications, 106: 276-285.
Specific fluorogenic substrates for neprilysin (neutral endopeptidase, EC 3.4.24.11) which are highly resistant to serine- and metalloproteases
Correspondence and Footnotes
Publication Dates
-
Publication in this collection
07 Oct 1998 -
Date of issue
Oct 1997
History
-
Accepted
19 Aug 1997 -
Received
11 Apr 1997