Abstract
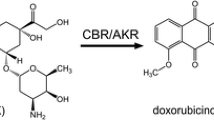
Carbonyl reductase (CR) catalyzes the nicotinamide adenine dinucleotide phosphate (NADPH)-dependent reduction of several carbonyls. Anthracyclines used to treat cancer are reduced by CR at the C13 carbonyl and the resulting metabolites are implicated in the cardiotoxicity associated with anthracycline therapy. CR also is believed to have a role in detoxifying quinones, raising the question whether CR catalyzes reduction of anthracycline quinones. Steadystate kinetic studies were done with several anthraquinone-containing compounds, including 13-deoxydoxorubicin and daunorubicinol, which lack the C13 carbonyl, thus unmasking the anthraquinone for study. kcat and kcat/Km values for 13-deoxydoxorubicin and daunorubicinol were nearly identical, indicating that that the efficiency of quinone reduction was unaffected by the differences at the C13 position. kcat and kcat/Km values were much smaller for the analogs than for the parent compounds, suggesting that the C13 carbonyl is preferred as a substrate over the quinone. CR also reduced structurally related quinone molecules with less favorable catalytic efficiency. Modeling studies with doxorubicin and carbonyl reductase revealed that methionine 234 sterically hinder the rings adjacent to the quinone, thus accounting for the lower catalytic efficiency. Reduction of the anthraquinones may further define the role of CR in anthracycline metabolism and may influence anthracycline cytotoxic and cardiotoxic mechanisms.
Similar content being viewed by others
References
Young, R. C., Ozols, R. F., and Myers, C. E. (1981). The anthracycline antineoplastic drugs. N. Engl. J. Med. 305: 139–153.
Cortes, E. P., Lutman, G., Wanka, J., Wang, J. J., Pickren, J., Wallace, J., and Holland, J. F. (1975). Adriamycin (NSC-123127) cardiotoxicity: a clinico-pathologic correlation. Cancer Chemother. Rep. 6:215–255.
Minow, R. B. R. and Gottleeb, J. (1975). Adriamycin (NSC-123127) cardiomyopathy-an overview with determination of risk factors. Cancer Chemother. Rep. 6:195–210.
Lipshultz, S. E., Colan, S. D., Gelber, R. D., Perez-Atyde, A. R., Sallan, S. E., and Sanders, S. P. (1991). Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N. Engl. J. Med. 324:808–815.
Olson, R. D. and Mushlin, P. S. (1990). Doxorubicin cardiotoxicity: analysis of prevailing hypotheses. FASEB J. 4: 3076–3086.
Boucek, R. J. Jr., Buck, S. H., Scott, F., Oquist, N. L., Fleischer, S., and Olson, R. D. (1993). Anthracycline-induced tension in permeabilized cardiac fibers: Evidence for the activation of the calcium release channel of sarcoplasmic reiculum. J. Mol. Cell. Cardiol. 25:249–259.
Boucek, R. J. Jr., Olson, R. D., Brenner, D. E., Ogunbunmi, E. M., Inui, M., and Fleischer, S. (1987). The major metabolite of doxorubicin is a potent inhibitor membrane associated ion pumps: a correlative study of cardiac muscle with isolated membrane fractions. J. Biol. Chem. 262:15,851–15,856.
Olson, R. D., Mushlin, P. S., Brenner, D. E., Fleischer, S., Cusack, B. J., Chang, B. K., and Boucek, R. J. Jr. (1988). Doxorubicin cardiotoxicity may be caused by its metabolite, doxorubicinol. Proc. Natl. Acad. Sci. USA 85:3585–3589.
Cusack, B. J., Mushlin, P. S., Voulelis, L. D., Li, X., Boucek, R. J., Jr., and Olson, R. D. (1993). Daunorubicin-induced cardiac injury in the rabbit: a role for daunorubicinol?. Toxicol. Appl. Pharmacol. 118:177–185.
Mushlin, P. S., Cusack, B. J., Boucek, R. J. Jr., Andrejuk, T., Li, X., and Olson, R. D. (1993). Time-related increases in cardiac concentrations of doxorubicinol could interact with doxorubicin to depress myocardial contractile function. Br. J. Pharm. 110:975–982.
Minotti, G., Cavaliere, A. F., Mordente, A., Rossi, M., Schiavello, R., Zamparelli, R., and Possati, R. (1995). Secondary alcohol metabolites mediate iron delocalization in cytosolic fractions of myocardial biopsies exposed to anticancer anthracyclines. J. Clin. Invest. 95:1595–1605.
Minotti, G., Menna, P., Salvatorelli, E., Cairo, G., and Gianni, L. (2004). Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 56:185–229.
Olson, R. D., Li, X., Palade, P., Shadle, S. E., Mushlin, P. S., Gambliel, H. A., et al. (2000). Sarcoplasmic reticulum calcium release is stimulated and inhibited by daunorubicin and daunorubicinol. Toxicol. Appl. Pharmacol. 169: 168–176.
Stewardt, D. J., Grewaal, D., Green, R. M., Mikael, N., Goel, R., Montpetit, V. A. J., and Redmond, M. D. (1993). Concentrations of doxorubicin and its metabolites in human autopsy heart and other tissues. Anticancer Res. 13:1945–1952.
Pollakis, G., Goormaghtigh, E., and Ruysschaert, J.-M. (1983). Role of the quinone structure in the mitochondrial damage induced by antitumor anthracyclines: comparison of adriamycin and 5-iminodaunorubicin. FEBS Lett. 155: 267–272.
Doroshow, J. (1983). Anthracycline antibiotic-stimulated superoxide, hydrogen peroxide, and hydroxyl radical production by NADH dehydrogenase. Cancer Res. 43:4543–4551.
Davies, K. J. A. and Doroshow, J. H. (1986). Redox cycling of anthracyclines by cardiac mitochondria: 1. Anthracycline radical formation by NADH dehydrogenase. J. Biol. Chem. 261:3060–3067.
Shadle, S. E., Bammel, B. P., Cusack, B. J., Knighton, R. A., Olson, S. J., Mushlin, P. S., and Olson, R. D. (2000). Daunorubicin cardiotoxicity: evidence for the importance of the quinone moiety in a free-radical-independent mechanism. Biochem. Pharmacol. 60:1435–1444.
Mordente, A., Meucci, E., Martorana, G. E., Giardina, B., and Minotti, G. (2001). Human heart cytosolic reductases and anthracycline cardiotoxicity. IUBMB Life 52:83–88.
Minotti, G., Cairo, G., and Monti, E. (1999). Role of iron in anthracycline cardiotoxicity: new tunes for an old song? FASEB J. 13:199–211.
Forrest, G. L. and Gonzalez, B. (2000). Carbonyl reductase. Chemico-Biol. Inter. 129:21–40.
Felsted, R. L. and Bachur, N. R. (1980). Mammalian carbonyl reductases. Drug. Metab. Rev. 11:1–60.
Wermuth, B., Platts, K. L., Seidel, A., and Oesch, F. (1986). Carbonyl reductase provides the enzymatic basis of quinone detoxification in man. Biochem. Pharmacol. 35:1277–1282.
Wermuth, B. (1981). Purication and properties of an NADPH-dependent carbonyl reductase from human brain. J. Biol. Chem. 256:1206–1213.
Bohren, K. M., von Wartburg, J. P., and Wermuth, B. (1987). Kinetics of carbonyl reductase from human brain. Biochem. J. 244:165–171.
Olson, L. E., Bedja, D., Alvey, S. J., Cardounel, A. J., Gabrielson, K. L., and Reeves, R. H. (2003). Protection from doxorubicin-induced cardiac toxicity in mice with a null allele of carbonyl reductase 1. Cancer Res. 63:6602–6606.
Forrest, G. L., Gonzalez, B., Tseng, W., Li, X., and Mann, J. (2000). Human carbonyl reductase overexpression in the heart advances the development of doxorubicin-induced cardiotoxicity in transgenic mice. Cancer Res. 60:5158–5164.
Minotti, G., Ronchi, R., Salvatorelli, E., Menna, P., and Cairo, G. (2001). Doxorubicin irreversibly inactivates iron regulatory proteins 1 and 2 in cardiomyocytes: evidence for distinct metabolic pathways and implications for ironmediated cardiotoxicity of antitumor therapy. Cancer Res. 61:8422–8428.
Takanashi, S. and Bachur, N. R. (1976). Adriamycin metabolism in man: evidence from urinary metabolism. Drug Metab. Dispos. 4:79–87.
Forrest, G. L., Akman, S., Doroshow, J., Rivera, H., and Kaplan, W. D. (1991). Genomic sequence and expression of a cloned human carbonyl reductase gene with daunorubicin reductase activity. Mol. Pharmacol. 40:502–507.
Bohren, K. M., Wermuth, B., Harrison, D., Dagmar, R., Petsko, G. A., and Gabbay, K. H. (1994). Expression, crystallization and preliminary crystallographic analysis of human carbonyl reductase. J. Mol. Biol. 244:659–664.
Bradford, M. M. (1976). A rapid and sensitive for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254.
Windholz, M., Budavari, S., Stroumtsos, L. Y., and Fertig, M. N. (1976). The Merck Index: An encyclopedia of chemical and drugs. 9th Edition, Merck & Co., Rahway, NJ.
Wermuth, B. (1985). Aldo-keto reductases, in Enzymology of Carbonyl Metabolism: Aldehyde Dehydrogenase, Aldo/Keto Reductase, and Alcohol Dehydrogenase (Flynn, T. G. and Weiner, H., ed.), Alan R. Liss, New York: pp. 209–230.
Cleland, W. W. (1979). Statistical analysis of enzyme kinetic data. Meth. Enz. 63:103–138.
Kong, J., White, C. A., Krylov, A. I., Sherrill, C. D., Adamson, R. D., Furlani, T. R., et al. (2000). Q-Chem 2.0: a highperformance abinitio electronic structure program package. J. Computational Chem. 21:1532–1548.
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., et al. (2004). Gaussian 03, Revision C.02. Gaussian, Inc., Wallingford, CT.
Ghosh, D., Sawicki, M., Pletnev, V., Erman, M., Ohno, S., Nakajin, S., and Duax, W. L. (2001). Porcine carbonyl reductase: structural basis for a functional monomer in short chain dehydrogenases/reductases. J. Biol. Chem. 276: 18,457–18,463.
Jones, T. A., Cowan, S., Zou, J.-Y., and Kjeldgaard, M. (1991). Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A. 47:110–119.
Lipscomb, L. A., Peek, M. E., Zhou, F. X., Bertrand, J. A., VanDerveer, D., and Williams, L. D. (1994). Water ring structure at DNA interfaces: hydration and dynamics of DNA-anthracycline complexes. Biochemistry 33:3649–3659.
Ax, W., Soldan, M., Koch, L., and Maser, E. (2000). Development of daunorubicin resistance in tumour cells by induction of carbonyl reduction. Biochem. Pharmacol. 59:293–300.
Gonzalez, B., Akman, S., Doroshow, J., Rivera, H., Kaplan, W. D., and Forrest, G. L. (1995). Protection against daunorubicin cytotoxicity by expression of a cloned human carbonyl reductase cDNA in K562 leukemia cells. Cancer Res. 55:4646–4650.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Slupe, A., Williams, B., Larson, C. et al. Reduction of 13-deoxydoxorubicin and daunorubicinol anthraquinones by human carbonyl reductase. Cardiovasc Toxicol 5, 365–376 (2005). https://doi.org/10.1385/CT:5:4:365
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/CT:5:4:365