-
PDF
- Split View
-
Views
-
Cite
Cite
Tsukasa Nakamura, Chifuyu Ushiyama, Shingo Suzuki, Masanori Hara, Noriaki Shimada, Isao Ebihara, Hikaru Koide, Urinary excretion of podocytes in patients with diabetic nephropathy, Nephrology Dialysis Transplantation, Volume 15, Issue 9, September 2000, Pages 1379–1383, https://doi.org/10.1093/ndt/15.9.1379
Close - Share Icon Share
Abstract
Background. Detection of podocytes in the urinary sediments of children with glomerulonephritis has been shown to indicate severe injury to the podocytes. The aim of the present study was to determine whether podocytes are present in the urine sediments of adult patients with diabetes with and without nephropathy and whether trandolapril is effective for podocyte injury.
Methods. Fifty diabetic patients (10 with normoalbuminuria, 15 with microalbuminuria, 15 with macroalbuminuria and 10 with chronic renal failure) and 10 healthy controls were studied. Urinary podocytes were examined by immunofluorescence using monoclonal antibodies against podocalyxin, which is present on the surface of podocytes. In addition, we studied plasma metalloproteinase (MMP)‐9 concentrations in all patients.
Results. Urinary podocytes were absent in healthy controls, diabetic patients with normoalbuminuria and diabetic patients with chronic renal failure. Podocytes were detected in the urine of eight diabetic patients with microalbuminuria (53%) and of 12 patients with macroalbuminuria (80%). The number of podocytes in the urine of patients with macroalbuminuria was significantly greater than in patients with microalbuminuria (P<0.01). However, there was no relationship between urinary albumin excretion and urinary podocytes. In addition, plasma MMP‐9 concentrations were significantly correlated with the number of urinary podocytes (P<0.01). Twelve diabetic patients with macroalbuminuria and eight patients with microalbuminuria who had urinary podocytes were treated with the angiotensin‐converting enzyme inhibitor trandolapril. Urinary albumin excretion, the number of podocytes and plasma MMP‐9 concentrations were reduced by the trandolapril treatment.
Conclusions. Podocytes in the urine may be a useful marker of disease activity in diabetic nephropathy. Trandolapril may be effective for podocyte injury.
Introduction
Podocytes are thought to support the glomerular basement membrane, and the integrity of these cells and structural preservation of their foot processes are important in glomerular filtration [1]. Podocytes are target cells in the glomerulus for various stimuli, and podocyte injury occurs to varying degrees in pathological conditions. Podocytes are located on the outer surface of the glomerular basement membrane; thus, injured podocytes or related structures appear in the urine if injury to the cell is serious enough. A glomerular epithelial cell antigen, podocalyxin, has been found in the urine of patients with glomerular disease [2,3]. Hara et al. detected podocalyxin‐marked podocytes in urinary sediments of patients with various forms of glomerulonephritis and reported that the number of podocytes in the urine was a direct indication of the degree of glomerular epithelial cell injury in children with glomerular disease [4,5]. Urinary podocytes recently were described as a marker for estimating the severity of active glomerular injury and a predictor of disease progression [6]. However, little is known about urinary podocyte excretion in diabetic nephropathy in adults.
Metalloproteinase (MMP)‐9 has a broad substrate specificity for native collagens, proteoglycans and elastin [7]. MMP‐9 has been identified in a variety of tissues and body fluids under pathophysiological conditions including smoking, cancers and polycystic kidney diseases [8–10]. We recently have reported that an increase in plasma concentrations preceded the development of microalbuminuria in diabetic patients [11].
It is well known that the renin–angiotensin system is stimulated in diabetic patients with nephropathy and that angiotensin II influences the synthesis of glomerular proteins [12]. We and others have reported that angiotensin‐converting enzyme (ACE) inhibitors are therapeutically effective in non‐insulin‐dependent diabetic patients with nephropathy [11,13,14]. Trandolapril is a new, orally active, long‐lasting non‐sulfhydryl ACE inhibitor ideal for a once‐daily regimen [15]. The aim of the present study was to determine whether urinary podocytes are detected in patients with diabetes and whether trandolapril affects the number of podocytes in the urine.
Materials and methods
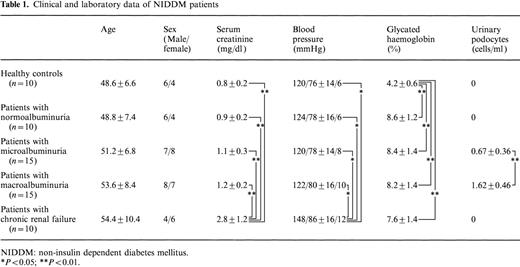
Ten diabetic patients with normoalbuminuria (group A), 15 diabetic patients with microalbuminuria (group B), 15 diabetic patients with macroalbuminuria (group C) and 10 patients with chronic renal failure (group D) with non‐insulin‐dependent diabetes mellitus (NIDDM) and 10 healthy controls were recruited for the present study and gave informed consent. NIDDM was diagnosed according to World Health Organization criteria. Insulin was not necessary for at least 3 years from the onset of diabetes, and body weight was stable in the last years (2.8±0.1%). There was no malignancy or history of heart disease, cerebrovascular disease, liver disease or collagen disease in patients in groups A–C, and they had normal blood pressure. Clinical and laboratory data of subjects is shown in Table 1. After an overnight fast, blood was drawn from an antecubital vein for measurement of glucose, glycated haemoglobin and serum creatinine concentration. Plasma glucose concentration was determined by the glucose oxidase method, and plasma‐glycated haemoglobin concentration was measured by spectrophotometric assay (Bio‐Rad, Richmond, VA; normal range, 3.5–6.5%). Normal blood pressure values (systolic blood pressure <140 mmHg, diastolic blood pressure <90 mmHg) were observed on two consecutive examinations [14]. On the basis of median urinary albumin excretion (UAE) from at least three consecutive 4‐h urine collections (taken in the morning), patients were classified as follows; microalbuminuria was defined as a median UAE of 20–200 μg/min and macroalbuminuria as UAE>200 μg/min. Patients with renal dysfunction (serum creatinine >1.5 mg/dl, creatinine clearance<80 ml/min) were included in group D.
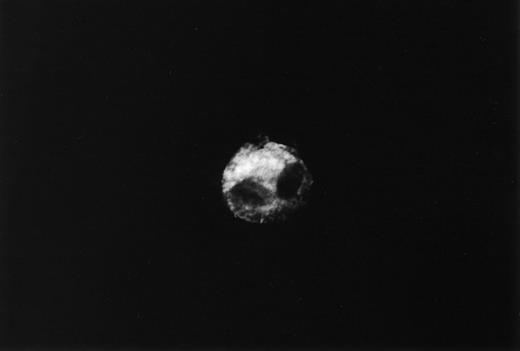
Immunofluorescent staining of urinary podocytes was performed as previously described [4,6]. Urine sediments were cytospun on glass slides and incubated with anti‐human podocalyxin monoclonal antibody PHM5 (Australian Monoclonal Development, Australia). This antibody reacts with a 165–170 kDa band of podocalyxin, as demonstrated by immunoblot analysis, on podocytes in human kidney immunohistology sections [6]. This antibody stained glomerular epithelial cells intensely and endothelial cells faintly in normal kidney sections. The staining was virtually identical in all these glomeruli regardless of the type of glomerular diseases. However, the glomerular epithelial cells adjacent to adhesions or crescents were not stained with this antibody [4]. Western blot analysis of this antibody was performed by Hara et al. [4]. They also reported the fine structure of the podocalyxin‐positive cells in the urinary sediments by immunoelectron microscopy using this antibody [4]. The surface of these cells has many short villous structures visible with labelled anti‐podocalyxin antibody. The diameter of the villous structures is similar to that of fine granules identified by immunofluorescence microscopy. After washing, slides were incubated with fluorescein isothiocyanate‐labelled F(ab′)2 fragments of affinity‐purified anti‐mouse IgG (Cappel, USA). Slides were washed and examined under an immunofluorescence microscope. The nuclei of cells were counterstained with ethidium bromide. We reported previously that podocalyxin was present in the urinary sediments of patients with glomerular diseases in the following three patterns: cast, fine granules and entire cells [4]. In the present study, we measured entire cells, not cell fragments, in the urine.
To measure the plasma concentrations of MMP‐9, a one‐step sandwich enzyme immunoassay using two monoclonal antibodies was used as previously described [16]. The sensitivity of the assay system was 0.24 ng/ml (2.2 pg/assay), and linearity was obtained from 0.24 to 250 ng/ml (2.2–2280 pg/assay). The intra‐assay coefficient of variation ranged from 1.8 to 3.9%, while the inter‐assay coefficient ranged from 1.8 to 6.2% [8,9,11,16].
Patients with microalbuminuria or macroalbuminuria who demonstrated urinary podocytes were treated with oral trandolapril (Chugai Co., Ltd, Tokyo, Japan; 1 mg/day) for 2 months, and urinary podocytes and plasma MMP‐9 concentrations were examined after treatment.
Data are expressed as mean ±standard deviation. Statistical analyses were performed using analysis of variance and the Mann–Whitney U‐test.
Results
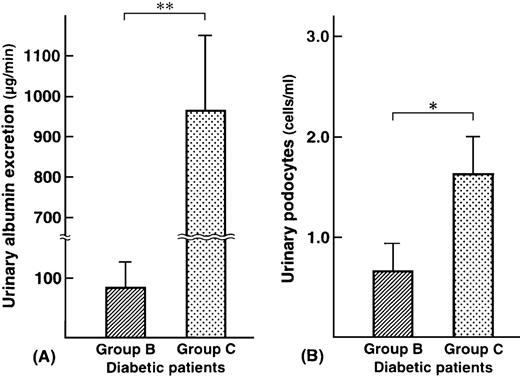
Podocalyxin‐positive cells are shown in Figure 1. Podocalyxin‐positive cells were detected in the urinary sediments of eight out of 15 patients with microalbuminuria (53%) (range: 0.8–1.9 cells/ml) and 12 out of 15 patients with macroalbuminuria (80%) (range: 0.8–5.3 cells/ml). Urinary albumin excretion and urinary podocytes are shown in Figure 2. There was no relationship between the degree of albuminuria and the number of podocalyxin‐positive cells. In group B, urinary albumin excretion in patients who had positive urinary podocytes (mean: 53.8 μg/min) was less than that in patients who had negative urinary podocytes (mean: 126.3 μg/min). Patient number 1 in group B had the largest number of urinary podocytes, but the least urinary albumin excretion. In group C, urinary albumin in patients who had positive urinary podocytes (mean: 936.7 μg/min) was less than that in patients who had negative urinary podocytes (mean: 1073.3 μg/min). There was also no relationship between blood glucose level or blood pressure and the number of podocalyxin‐positive cells (P=0.09 and 0.08, respectively; not significant). In addition, there was no relationship between glycaemic control, diabetic duration and the number of urinary podocytes (P=0.08 and 0.10, respectively; not significant). None of the diabetic patients with normoalbuminuria or with chronic renal failure and none of the healthy controls had urinary podocytes.
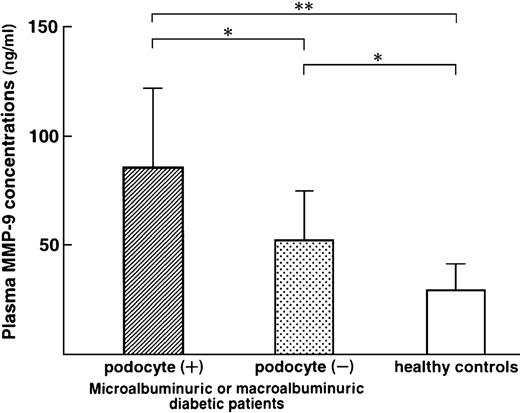
Plasma MMP‐9 concentrations in 20 diabetic patients with detectable urinary podocyte excretion (microalbuminuric patients, n=8 and macroalbuminuric patients, n=12: 86.4±36.6 ng/ml) were significantly higher than those in 10 diabetic patients with undetectable urinary podocyte excretion (microalbuminuric patients, n=7 and macroalbuminuric patients, n=3: 52.6±22.8 ng/ml, P<0.05) and healthy controls (30.6±10.8 ng/ml, P<0.01) (Figure 3).
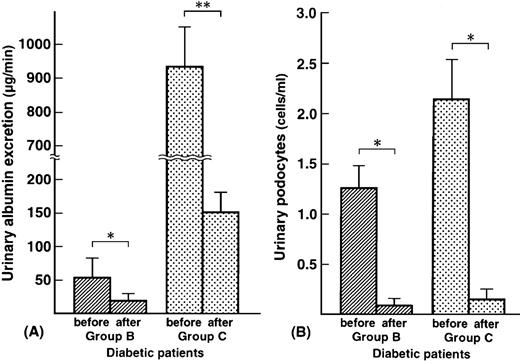
Urinary albumin excretion and urinary podocytes before and 2 months after trandolapril treatment in eight diabetic patients with microalbuminuria and 12 patients with macroalbuminuria are shown in Figure 4. Trandolapril reduced urinary albumin excretion in all patients (group B, from 53.8±26.2 μg/min to 17.3±8.2 μg/min, P<0.01; group C, from 936.7± 282.6 μg/min to 151.3±36.2 μg/min, P<0.001). Trandolapril also reduced the number of podocytes in the urine (group B; mean, from 1.25 cells/ml to 0.08 cells/ml, P<0.01, group C; mean, from 2.03 cells/ml to 0.13 cells/ml, P<0.01). Trandolapril reduced plasma MMP‐9 concentrations in 20 diabetic patients with detectable urinary podocyte excretion (from 86.4±36.6 ng/ml to 54.8±22.6 ng/ml, P<0.05). Trandolapril did not affect serum creatinine and glycated haemoglobin levels in groups B and C. Trandolapril reduced blood pressure from 120± 14 mmHg to 108±8 mmHg in group B (P<0.05) and from 122±16 mmHg to 110±10 mmHg in group C (P<0.05).
Urinary albumin excretion (μg/min) (A) and urinary podocytes (cells/ml) (B) in diabetic patients. Group B: microalbuminuric patients; group C: macroalbuminuric patients. *P<0.01; **P<0.001.
Plasma metalloproteinase (MMP)‐9 concentrations (ng/ml) in diabetic patients with detectable urinary podocytes, in diabetic patients with undetectable urinary podocytes and in healthy controls. *P<0.05; **P<0.01.
Urinary albumin excretion (μg/min) (A) and urinary podocytes (cells/ml) (B) in diabetic patients before and after 2 month of trandolapril treatment. Group B: microalbuminuric patients; group C: macroalbuminuric patients. *P<0.01; **P<0.001.
Discussion
In contrast to urine samples from diabetic patients with normoalbuminuria, diabetic patients with chronic renal failure and healthy controls, podocytes were detected in urinary sediments obtained from diabetic patients with microalbuminuria and diabetic patients with macroalbuminuria. Daniels reported that in conjunction with the glomerular basement membrane, the glomerular podocyte plays an important role in glomerular filtration [1]. The loss of podocytes from the glomerular basement membrane impairs the glomerular filtration process.
Analyses of kidney biopsies from Pima Indians with NIDDM demonstrated that subjects with clinical nephropathy exhibited broadening of podocyte foot processes associated with a reduction in the number of podocytes per glomerulus and an increase in the surface area covered by remaining podocytes, suggesting that podocyte loss contributes to the progression of diabetic nephropathy [17]. Focal detachment of podocytes from the glomerular basement membrane may be associated with a decrease in α3β1 integrin at the podocyte basal plasma membrane, which occurs as early as 1 month after of hyperglycaemia [18]. It is possible to count the number of lost podocytes in the urine since podocytes are located in the urinary space of the glomerular capillary walls and, therefore, appear in the urine if they are lost from the glomerular basement membrane. Hara et al. reported that a considerable number of podocytes exists in the urine of children with various glomerular diseases [4,6]. However, little is known about adults with diabetic nephropathy. Since increased urinary albumin excretion could be the consequence of augmented intraglomerular capillary pressure, microalbuminuria could reflect intrinsic glomerular damage causing changes in glomerular barrier function [19]. Recently, Meyer et al. [20] reported that among glomerular morphological characteristics, the number of podocytes per glomerulus was the strongest predictor of renal disease progression in diabetics. Since podocytes are thought to be incapable of replication, podocyte loss or a low podocyte number per glomerulus contributes to the development and progression of glomerulosclerosis [17]. Therefore, the detection of podocytes in the urine sediments is a simple and non‐invasive procedure to examine podocyte injury.
In diabetic patients, there is a close association between microalbuminuria and epithelial dysfunction [21]. We previously have reported that an increase in plasma MMP‐9 preceded the occurrence of microalbuminuria in diabetic patients [11]. McMillan et al. [22] reported increased glomerular epithelial cell MMP‐9 expression to be correlated with the period of proteinuria in rat Heymann nephritis and that a direct link exists between glomerular epithelial cell proteolytic activities and loss of glomerular permselectivity. We speculated that one of the sources of increased plasma MMP‐9 levels may be epithelial cells, and observed that diabetic patients with urinary podocytes showed high plasma MMP‐9 concentrations (Figure 3).
ACE inhibitors decrease proteinuria and slow the progression of diabetic nephropathy by mechanisms that cannot be attributed solely to effective control of systemic hypertension. Some investigators have reported the efficacy of ACE inhibitors in reducing the risk of developing end‐stage renal disease in NIDDM patients [13,23]. Trandolapril is effective in diabetic nephropathy in rats and humans [24,25]. In the present study, trandolapril reduced urinary albumin excretion and plasma MMP‐9 concentrations, as well as urinary podocytes in patients with diabetic nephropathy. However, the precise mechanism by which ACE inhibitors act on urinary podocytes is still unknown. We are now studying the effect of other anti‐hypertensive drugs including calcium channel antagonists and angiotensin II receptor antagonists on urinary podocyte excretion in diabetic patients.
Unexpectedly, diabetic patients with chronic renal failure had no podocyte excretion in the urine. We recognized that urinary podocytes were absent in chronic renal failure patients with a variety of glomerular diseases such as IgA nephropathy, focal glomerulosclerosis, membranous nephropathy and membranoproliferative glomerulonephritis (unpublished data). These results suggest that the presence of urinary podocytes may be a marker of disease severity during the active phase of glomerular injury, but not during the chronic progressive phase of glomerular disease.
In summary, detection of podocytes in the urinary sediments by immunofluorescence indicates that glomerular epithelial cell injury occurs in patients with diabetic nephropathy. Trandolapril may have a beneficial effect on glomerular epithelial cell injury.
Correspondence and offprint requests to: Hikaru Koide, MD, Department of Medicine, Koto Hospital, 6‐8‐5 Ojima, Koto‐ku, Tokyo 136‐0072, Japan.
We are grateful to Mitsubishi Kagaku Bio‐Clinical Laboratories, Inc., Tokyo, Japan, for technical support. We thank Ms Yukiko Suzuki, Nephrology Unit, Misato Junshin Hospital, Saitama, for expert technical assistance.
References
Daniels BS. The role of the glomerular epithelial cell in the maintenance of the glomerular filtration barrier.
Hancock WW, Atkins RC. Monoclonal antobodies to human glomerular cells: a marker for glomerular epithelial cells.
Kerjaschki D, Poczewski H, Dekan G et al. Identification of a major sialoprotein in the glycocalyx of human visceral glomerular epithelial cells.
Hara M, Yamanaka T, Yanagihara T et al. Urinary excretion of podocalyxin indicates glomerular epithelial cell injuries in glomerulonephritis.
Hara M, Yanagihara T, Itoh M, Matsuno M, Kihara I. Immunohistochemical and urinary markers of podocyte injury.
Hara M, Yanagihara T, Takada T et al. Urinary excretion of podocytes reflects disease activity in children with glomerulonephritis.
Senior RM, Griffin GL, Fliazar CJ, Shapiro SD, Goldberg GI, Welgus HG. Human 92‐ and 72‐kilodalton type IV collagenases are elastase.
Torii A, Kodera Y, Uesaka K et al. Matrix metalloproteinase‐9 in human plasma has 96% specificity and 56% sensitivity for gastric cancer screening.
Nakamura T, Ebihara I, Shimada N, Koide H. Effect of cigarette smoking on plasma metalloproteinase‐9 concentration.
Nakamura T, Ushiyama C, Suzuki S, Ebihara I, Shimada N, Koide H. Elevation of serum levels of metalloproteinase‐1, tissue inhibitor of metalloproteinase‐1 and type IV collagen, and plasma levels of metalloproteinase‐9 in polycystic kidney disease.
Ebihara I, Nakamura T, Shimada N, Koide H. Increased plasma metalloproteinase‐9 concentrations precede development of microalbuminuria in non‐insulin‐dependent diabetes mellitus.
Hasslacher C, Kempe HP, Bostedt‐Kiesel A. ACE inhibitors and diabetic nephropathy: clinical and experimental findings.
Ravid M, Lang R, Rachmani R, Lisher M. Long‐term renoprotective effect of angiotensin‐converting enzyme inhibitor in non‐insulin‐dependent diabetes mellitus.
Ahmad J, Siddiqui MA, Ahmad H. Effective postponement of diabetic patients with microalbuminuria.
Bachhouse CI, Orofiamma B, Pauly NC, for the Investigator Study Group. Long‐term therapy with trandolapril, a new nonsulfhydryl ACE inhibitor, in hypertension: a multicenter international trial.
Fujimoto N, Hosokawa N, Iwata K, Shinya T, Okada Y, Hayakawa T. A one‐step sandwich enzyme immunoassay for inactive precursor and complexed forms of human matrix metalloproteinase‐9 using monoclonal antibodies.
Pagtalunan ME, Miller PL, Jumpimg‐Eagle S et al. Podocyte loss and progressive glomerular injury in type II diabetes.
Regoli M, Bendayan M. Alterations in the expression of the alpha 3 beta 1 integrin in certain membrane domains of the glomerular epithelial cells (podocytes) in diabetes mellitus.
Rodicio JL, Campo C, Ruilope LM. Microalbuminuria in essential hypertension.
Meyer TW, Benett PH, Nelson RG. Podocyte number predicts long‐term urinary albumin excretion in Pima Indians with type II diabetes and microalbuminuria.
Stehouwer CDA, Andres‐Fischer HR, van zkuijk AWR, Polak BCP, Donker AJM. Endothelial dysfunction precedes development of microalbuminuria in IDDM.
McMillan JI, Riordan JW, Couser WG, Pollock AS, Lovett DH. Characterization of a glomerular epithelial cell metalloproteinases as matrix metalloproteinase‐9 with enhanced expression in a model of membranous nephropathy.
Yokoyama H, Tomonaga O, Hirayama M et al. Predictors of the progression of diabetic nephropathy and the beneficial effect of angiotensin‐converting enzyme inhibitors in NIDDM patients.
Sassy‐Prigent C, Heudes D, Jouquey S et al. Morphometric detection of incipient glomerular lesions in diabetic nephropathy in rats. Protective effects of ACE inhibition.





Comments