-
PDF
- Split View
-
Views
-
Cite
Cite
Maria Wikström, Gunilla Thelander, Maria Dahlgren, Robert Kronstrand, An Accidental Fatal Intoxication with Methoxetamine, Journal of Analytical Toxicology, Volume 37, Issue 1, January/February 2013, Pages 43–46, https://doi.org/10.1093/jat/bks086
Close - Share Icon Share
Abstract
This paper reports an unintentional death involving the administration of methoxetamine [2-(3-methoxyphenyl)-2-(ethylamino)-cyclohexanone] and offers some reference values from living drug abusers. Methoxetamine is a new recreational drug with a similar structure to ketamine. The deceased was a 26-year-old male with a history of drug abuse; he was found lying on the floor in his apartment. Several “red-line” plastic bags were found, one of which was labeled “2-(3-methoxyphenyl)-2-(ethylamino)-cyclohexanone” and another labeled “Haze.” In four cases from living subjects with unknown doses, concentrations of methoxetamine were found from 0.13 to 0.49 µg/g. In three of the cases, the blood samples also contained natural or synthetic cannabinoids. In the autopsy case, a considerably higher concentration of methoxetamine, 8.6 µg/g, was found in femoral blood. In addition, tetrahydrocannabinol and the three different synthetic cannabinoids AM-694, AM-2201, and JWH-018, were present in femoral blood. The circumstances and the high femoral blood concentration of methoxetamine point toward an unintentional, acute fatal intoxication with methoxetamine, although the presence of the three synthetic cannabinoids may have contributed to the death.
Introduction
Methoxetamine, 2-(3-methoxyphenyl)-2-(ethylamino)-cyclohexanone, is a new recreational drug with a similar structure to ketamine. The chlorine atom at position 2 has been exchanged by a methoxy-group at position 3 on the phenyl ring and the N-alkyl chain has been extended by one carbon (Figure 1). Because it is still a legal substance in many countries, it is readily provided through the internet without legal issues. The drug first appeared in 2010 and is widely marketed as a substitute to ketamine; it has recently been reported by drug monitoring systems (1). Users of methoxetamine administer it orally, by snorting, rectally or by intramuscular injection. Doses up to 500 mg have been reported in cases of intoxications (2), but typical recreational doses range from 20 to 100 mg. The effects begin after 10 to 20 min, with a duration of 2 to 3 h (3). The longer duration compared to ketamine is thought to be attributed to the ethyl group on the nitrogen (3). Clinical effects and toxicological symptoms after administration of methoxetamine are euphoria, agitation, hallucinations, disorientation, confusion, vertigo, analgesia, numbness, anxiety, tachycardia, respiratory depression, nausea and vomiting in a dose-dependent manner (3–4). Few cases of methoxetamine abuse have been published in the scientific literature. In a case series of patients arriving at hospitals, it was demonstrated that acute methoxetamine-related toxicity is associated with a state of dissociation and sympathomimetic signs of tachycardia and hypertension (2, 4). Although there have been no pharmacological studies in humans or animals, it has been suggested that the mechanism of action for methoxetamine shares that of ketamine, through the N-ethyl-D-aspartate (NMDA) receptor antagonism and inhibition of dopamine reuptake. Ketamine, phencyclidine (PCP) and analogues of these substances are structurally related to arylcycloalkylamine compounds, primarily acting on the NMDA receptor as open channel blockers, and are classified as dissociative anesthetics. They are also dopamine D2-receptor agonists and interact with 5-HT2 receptors, µ and κ opioid receptors, σ receptors and muscarinic cholinergic receptors (5–6). Chronic treatment with ketamine has been reported to be associated with severe symptomatic urinary tract problems. It is believed that methoxetamine does not produce these adverse effects, and this fact is used as one of the advantages when marketing the drug (2).
Structures: 2-(3-methoxyphenyl)-2-(ethylamino)-cyclohexanone (methoxetamine) (A); ketamine (B).
This paper reports an unintentional death involving the administration of methoxetamine and offers some reference values from living drug abusers.
Autopsy Case
The deceased was a 26-year-old male with a history of drug abuse and depression, for which he was prescribed Venlafaxine. He was found lying on the floor in his apartment. Several “red-line” plastic bags were found, one of which was labeled “2-(3-methoxyphenyl)-2-(ethylamino)-cyclohexanone” and another labeled “Haze.” No information indicated suicide. A full autopsy was performed and the internal examination revealed pulmonary edema. Samples of femoral blood, urine and vitreous humor were collected according to Swedish regulations (7). Routine postmortem toxicology was performed in femoral blood using a targeted screening for pharmaceuticals and drugs of abuse with liquid chromatography time-of-flight technology (8), and positive findings were confirmed and quantified with a different technique. Ethanol was analyzed in femoral blood and urine using headspace gas chromatography (GC) (9). Methoxetamine was confirmed and quantified by liquid chromatography–tandem mass spectrometry (LC–MS-MS), as described in the following.
Reference concentrations from living subjects
Apart from the autopsy case, methoxetamine was identified and quantified in whole blood samples from four cases of petty drug offense in which internet drugs had been requested by the police. Samples were screened for internet drugs using a GC–MS scan method (10) and methoxetamine was identified. These positive findings were confirmed and quantified by LC–MS-MS, as described in the following. In three of the cases, no other information about the circumstances was available. In the fourth case, the subject was found at home. Upon the arrival of the police, he was unresponsive and a bag labeled “2-(3-methoxyphenyl)-2-(ethylamino)-cyclohexanone” was found in his room. Upon arrival at the hospital, the hospital staff obtained blood samples.
Experimental
Quantitation of methoxetamine in blood
Methoxetamine hydrochloride and the internal standard, ketamin-D4, were purchased from LGC Standards (London, UK). Sample preparation was performed using a single precipitation step. To 0.25 g blood was added 0.025 µg of ketamine-d4 as internal standard and 1.0 mL of 0.075% formic acid in acetonitrile–ethanol (90/10). After mixing and centrifugation (10 min each), 50 µL of the supernatant was transferred to a vial and mixed with 50 µL of 0.05% formic acid in 10 mM ammonium formiate (mobile phase A). Samples with concentrations above the highest calibrator were diluted with drug-free blood and re-analyzed.
The LC–MS-MS system consisted of a Waters Acquity ultra-performance LC (UPLC) with a binary solvent manager, sample manager and column manager (Waters, Milford, MA) connected to an API 4000 triple quadrupole instrument (Applied Biosystems/MDS Sciex, Stockholm, Sweden) equipped with an electrospray interface (Turbo V source, TurboIonSpray probe) operating in the multiple reaction monitoring (MRM) mode. Ion spray voltage was set to 5,500 V. Nitrogen was used as nebulizer gas (345 kPa), heater gas (517 kPa at 500°C), curtain gas (207 kPa) and collision-activated dissociation gas (set on 5). UPLC was performed using a 1.7 µm, 50 × 2.1 mm Acquity UPLC HSS-T3 C18 column (Waters), preceded by a 0.2 µm column filter (Waters), operated at 0.6 mL/min with a total run time of 3 min. Mobile phase A consisted of 0.05% formic acid in 10 mM ammonium formate and mobile phase B was 0.05% formic acid in methanol. The chromatographic system was run in a linear gradient from 30 to 75% phase B in 2 min, then increased to 98% phase B for 0.5 min, followed by a 0.5 min equilibration with 98% phase A. The injection volume was 2 µL and the column manager temperature was set to 60°C. Instrument control, integration and calculation were performed using Analyst 1.4.2 software. The final MRM method included three transitions 248/203, 248/121 and 248/175 for methoxetamine, and 242/129 for ketamine-d4 with a dwell time of 50 ms for each transition.
Validation for the analysis of methoxetamine in blood
Validation was performed as suggested by Peters et al. for methods used in case reports or single cases (11). Selectivity was investigated by analyzing nine postmortem blood samples of varying degrees of decomposition without the addition of internal standard, and one sample containing internal standard. The calibration model was investigated by the analysis of triplicates at seven levels; 0.05, 0.10, 0.50, 1.0, 2.0, 3.0 and 5.0 µg/g. Accuracy and precision was estimated by the analysis of five replicates at two levels, 0.10 and 2.5 µg/g.
Results and Discussion
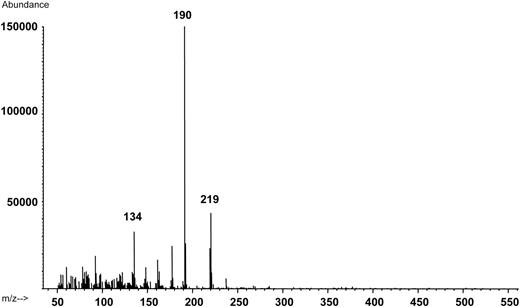
A GC–MS scan in positive mode from methoxetamine as a trifluoroacetic anhydride (TFAA)-derivative in Case 1 is shown in Figure 2. The fragmentation pattern is complicated, but a parallel can be drawn from the fragmentation pathways of ketamine, as proposed by Pieri et al. (12). They propose that the molecular ion loses carbon monoxide and forms an intermediate ion, from which further fragmentation occurs. A tentative explanation is that a methoxetamine intermediate (m/z 315) loses the derivative (m/z 315-96) and forms m/z 219. The base peak m/z 190 may be explained by loss of the ethyl group on the nitrogen. The fragment m/z 134 may represent the loss of the remaining carbons in the cyclohexylgroup.
Positive electrospray ionization mass spectra (m/z 50–550) from the peak corresponding to methoxetamine as a triflouraceticacid anhydride derivative in a blood extract from Case 4 (0.49 µg/g blood). The molecular ion (m/z 343) is not visible in the spectra.
The LC–MS-MS method showed no interfering peaks at the retention time of methoxetamine when investigating nine different postmortem blood samples, and the internal standard did not give any interfering peak. A calibration model using quadratic fit and weighting 1/X gave less than 10% deviation from the target value between 0.05 and 3.0 µg/g blood, which was determined as the working range. As presented in Table I, the within-day imprecision of the methoxetamine quantitation was 5.0% or less, with accuracy within 95% of the target value.
Accuracy and Precision for the Quantitation of Methoxetamine in Blood with LC–MS-MS (N = 5)
| . | Mean . | CV* (%) . | Accuracy (%) . |
|---|---|---|---|
| Low (0.1 µg/g) | 0.098 | 5.0 | 98 |
| High (2.5 µg/g) | 2.47 | 2.3 | 99 |
| . | Mean . | CV* (%) . | Accuracy (%) . |
|---|---|---|---|
| Low (0.1 µg/g) | 0.098 | 5.0 | 98 |
| High (2.5 µg/g) | 2.47 | 2.3 | 99 |
*Coefficient of variation.
Accuracy and Precision for the Quantitation of Methoxetamine in Blood with LC–MS-MS (N = 5)
| . | Mean . | CV* (%) . | Accuracy (%) . |
|---|---|---|---|
| Low (0.1 µg/g) | 0.098 | 5.0 | 98 |
| High (2.5 µg/g) | 2.47 | 2.3 | 99 |
| . | Mean . | CV* (%) . | Accuracy (%) . |
|---|---|---|---|
| Low (0.1 µg/g) | 0.098 | 5.0 | 98 |
| High (2.5 µg/g) | 2.47 | 2.3 | 99 |
*Coefficient of variation.
There are very few reference values of methoxetamine in blood. Wood et al. (2) reported concentrations between 0.09 and 0.2 µg/mL of serum in cases in which 200 to 500 milligrams had been ingested and in which the patients presented with hypertension and tachycardia in addition to dissociative and catatonic symptoms. In the cases from the four living subjects with unknown doses, concentrations were found from 0.13 to 0.49 µg/g (Table II). Case 1, with the highest concentration, was unresponsive when found by the police and later presented with incoherent and slurred speech. In three of the cases, the blood samples also contained natural or synthetic cannabinoids.2
Methoxetamine Concentrations in Whole Blood from Suspected Petty Drug Offenders
| Case . | Age . | Gender . | Methoxetamine (µg/g blood) . | Other findings . |
|---|---|---|---|---|
| 1 | 27 | Male | 0.21 | AM-2201, 0.001 µg/g |
| 2 | 57 | Male | 0.13 | — |
| 3 | 18 | Male | 0.13 | THC, 0.0004 µg/g AM-2201, 0.00008 µg/g |
| 4 | 22 | Male | 0.49 | Carboxy-THC, 0.002 µg/g |
| Case . | Age . | Gender . | Methoxetamine (µg/g blood) . | Other findings . |
|---|---|---|---|---|
| 1 | 27 | Male | 0.21 | AM-2201, 0.001 µg/g |
| 2 | 57 | Male | 0.13 | — |
| 3 | 18 | Male | 0.13 | THC, 0.0004 µg/g AM-2201, 0.00008 µg/g |
| 4 | 22 | Male | 0.49 | Carboxy-THC, 0.002 µg/g |
Methoxetamine Concentrations in Whole Blood from Suspected Petty Drug Offenders
| Case . | Age . | Gender . | Methoxetamine (µg/g blood) . | Other findings . |
|---|---|---|---|---|
| 1 | 27 | Male | 0.21 | AM-2201, 0.001 µg/g |
| 2 | 57 | Male | 0.13 | — |
| 3 | 18 | Male | 0.13 | THC, 0.0004 µg/g AM-2201, 0.00008 µg/g |
| 4 | 22 | Male | 0.49 | Carboxy-THC, 0.002 µg/g |
| Case . | Age . | Gender . | Methoxetamine (µg/g blood) . | Other findings . |
|---|---|---|---|---|
| 1 | 27 | Male | 0.21 | AM-2201, 0.001 µg/g |
| 2 | 57 | Male | 0.13 | — |
| 3 | 18 | Male | 0.13 | THC, 0.0004 µg/g AM-2201, 0.00008 µg/g |
| 4 | 22 | Male | 0.49 | Carboxy-THC, 0.002 µg/g |
In the autopsy case, a considerably higher concentration of methoxetamine, 8.6 µg/g, was found in femoral blood. In addition, therapeutic concentrations of venlafaxine (0.3 µg/g) and O-desmethylvenlafaxine (0.4 µg/g) were found in addition to tetrahydrocannabinol (0.001 µg/g). Three different synthetic cannabinoids were also present in femoral blood: AM-694 (0.00009 µg/g), AM-2201 (0.0003 µg/g) and JWH-018 (0.00005 µg/g). There is a paucity of data regarding concentrations of these compounds in humans, and therefore, it is difficult to evaluate whether their presence may have contributed to the death in this case. Synthetic cannabinoids were detected also in two of the living users. This suggests that it is common to simultaneously use different internet drugs, which might increase the risk of adverse effects.
Methoxetamine is sold in quantities up to 20 grams, although the oral dose recommended at the websites is only approximately 20–100 mg. Furthermore, a delayed effect has been reported, which might cause the user to ingest another dose, thinking the first one was insufficient (13). These two issues might increase the risk of accidental overdose.
At present, methoxetamine is legal in many countries and sold through the internet without any restrictions. Apart from the cases reported here, an additional 11 cases have presented with urine samples positive for methoxetamine during the first four months of 2012. Reports of toxicity and drug-related deaths will aid legislators to make decisions based on facts that will benefit society. To this end, the Swedish regulators have decided to schedule methoxetamine as an illicit drug, effective from May 1st, 2012 (14).
Conclusions
In conclusion, the circumstances and the high femoral blood concentration of methoxetamine point toward an unintentional acute fatal intoxication with methoxetamine, although it cannot be ruled out that the presence of three synthetic cannabinoids may have contributed to the death.