Abstract
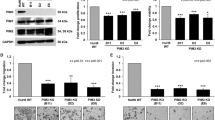
In spite of the established knowledge of the genetic alterations responsible for cancer onset, the genes promoting and maintaining the invasive/metastatic phenotype are still elusive. The MET proto-oncogene, encoding the tyrosine kinase receptor for hepatocyte growth factor (HGF), senses unfavorable micro-environmental conditions and drives cell invasion and metastasis. MET overexpression, often induced by tumor hypoxia, leads to constitutive activation of the receptor and correlates with poor prognosis. To establish the role of MET in different phases of tumor progression, we developed an inducible lentiviral delivery system of RNA interference. Silencing the endogenous MET gene, overexpressed in tumor cells, resulted in (i) impairment of the execution of the full invasive growth program in vitro, (ii) lack of tumor growth and (iii) decreased generation of experimental metastases in vivo. Notably, silencing MET in already established metastases led to their almost complete regression. This indicates that persistent expression of the MET oncogene is mandatory until the advanced phases of cancer progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bardelli A, Basile ML, Audero E, Giordano S, Wennstrom S, Menard S et al. (1999). Concomitant activation of pathways downstream of Grb2 and PI 3-kinase is required for MET-mediated metastasis. Oncogene 18: 1139–1146.
Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF . (2003). Met, metastasis, motility and more. Nat Rev Mol Cell Biol 4: 915–925.
Boccaccio C, Sabatino G, Medico E, Girolami F, Follenzi A, Reato G et al. (2005). The MET oncogene drives a genetic programme linking cancer to haemostasis. Nature 434: 396–400.
Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L et al. (1992). Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol 119: 629–641.
Chambers AF, Groom AC, MacDonald IC . (2002). Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2: 563–572.
Chin L, Tam A, Pomerantz J, Wong M, Holash J, Bardeesy N et al. (1999). Essential role for oncogenic Ras in tumour maintenance. Nature 400: 468–472.
Christensen JG, Schreck R, Burrows J, Kuruganti P, Chan E, Le P et al. (2003). A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res 63: 7345–7355.
Comoglio PM, Trusolino L . (2002). Invasive growth: from development to metastasis. J Clin Invest 109: 857–862.
Corbel SY, Rossi FM . (2002). Latest developments and in vivo use of the Tet system: ex vivo and in vivo delivery of tetracycline-regulated genes. Curr Opin Biotechnol 13: 448–452.
Corso S, Comoglio PM, Giordano S . (2005). Cancer therapy: can the challenge be MET? Trends Mol Med 11: 284–292.
Danilkovitch A, Zbar B . (2002). Dysregulation of Met receptor tyrosine kinase activity in invasive tumors. J Clin Invest 109: 863–867.
Di Renzo MF, Olivero M, Giacomini A, Porte H, Chastre E, Mirossay L et al. (1995a). Overexpression and amplification of the met/HGF receptor gene during the progression of colorectal cancer. Clin Cancer Res 1: 147–154.
Di Renzo MF, Olivero M, Martone T, Maffe A, Maggiora P, Stefani AD et al. (2000). Somatic mutations of the MET oncogene are selected during metastatic spread of human HNSC carcinomas. Oncogene 19: 1547–1555.
Di Renzo MF, Poulsom R, Olivero M, Comoglio PM, Lemoine NR . (1995b). Expression of the Met/hepatocyte growth factor receptor in human pancreatic cancer. Cancer Res 55: 1129–1138.
Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM et al. (2001). Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 344: 1031–1037.
Felsher DW, Bishop JM . (1999). Reversible tumorigenesis by MYC in hematopoietic lineages. Mol Cell 4: 199–207.
Fidler IJ . (2003). The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 3: 453–458.
Fixman ED, Naujokas MA, Rodrigues GA, Moran MF, Park M . (1995). Efficient cell transformation by the Tpr-Met oncoprotein is dependent upon tyrosine 489 in the carboxy-terminus. Oncogene 10: 237–249.
Follenzi A, Ailles LE, Bakovic S, Geuna M, Naldini L . (2000). Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat Genet 25: 217–222.
Frisch SM, Screaton RA . (2001). Anoikis mechanisms. Curr Opin Cell Biol 13: 555–562.
Galimi F, Cottone E, Vigna E, Arena N, Boccaccio C, Giordano S et al. (2001). Hepatocyte growth factor is a regulator of monocyte-macrophage function. J Immunol 166: 1241–1247.
Gambarotta G, Boccaccio C, Giordano S, Ando M, Stella MC, Comoglio PM . (1996). Ets up-regulates MET transcription. Oncogene 13: 1911–1917.
Gao CF, Vande Woude GF . (2005). HGF/SF-Met signaling in tumor progression. Cell Res 15: 49–51.
Giordano S, Corso S, Conrotto P, Artigiani S, Gilestro G, Barberis D et al. (2002). The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol 4: 720–724.
Graziani A, Gramaglia D, Cantley LC, Comoglio PM . (1991). The tyrosine-phosphorylated hepatocyte growth factor/scatter factor receptor associates with phosphatidylinositol 3-kinase. J Biol Chem 266: 22087–22090.
Grotegut S, von Schweinitz D, Christofori G, Lehembre F . (2006). Hepatocyte growth factor induces cell scattering through MAPK/Egr-1-mediated upregulation of Snail. EMBO J 25: 3534–3545.
Gunther EJ, Moody SE, Belka GK, Hahn KT, Innocent N, Dugan KD et al. (2003). Impact of p53 loss on reversal and recurrence of conditional Wnt-induced tumorigenesis. Genes Dev 17: 488–501.
Gupta GP, Massague J . (2006). Cancer metastasis: building a framework. Cell 127: 679–695.
Huettner CS, Zhang P, Van Etten RA, Tenen DG . (2000). Reversibility of acute B-cell leukaemia induced by BCR-ABL1. Nat Genet 24: 57–60.
Ivan M, Bond JA, Prat M, Comoglio PM, Wynford-Thomas D . (1997). Activated ras and ret oncogenes induce over-expression of c-met (hepatocyte growth factor receptor) in human thyroid epithelial cells. Oncogene 14: 2417–2423.
Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C et al. (2002). Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med 346: 645–652.
Kong-Beltran M, Stamos J, Wickramasinghe D . (2004). The Sema domain of Met is necessary for receptor dimerization and activation. Cancer Cell 6: 75–84.
Lee JH, Han SU, Cho H, Jennings B, Gerrard B, Dean M et al. (2000). A novel germ line juxtamembrane Met mutation in human gastric cancer. Oncogene 19: 4947–4953.
Lengauer C, Kinzler KW, Vogelstein B . (1998). Genetic instabilities in human cancers. Nature 396: 643–649.
Maulik G, Shrikhande A, Kijima T, Ma PC, Morrison PT, Salgia R . (2002). Role of the hepatocyte growth factor receptor, c-Met, in oncogenesis and potential for therapeutic inhibition. Cytokine Growth Factor Rev 13: 41–59.
Michieli P, Mazzone M, Basilico C, Cavassa S, Sottile A, Naldini L et al. (2004). Targeting the tumor and its microenvironment by a dual-function decoy Met receptor. Cancer Cell 6: 61–73.
Nowell PC . (1976). The clonal evolution of tumor cell populations. Science 194: 23–28.
Patane S, Avnet S, Coltella N, Costa B, Sponza S, Olivero M et al. (2006). MET overexpression turns human primary osteoblasts into osteosarcomas. Cancer Res 66: 4750–4757.
Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM . (2003). Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 3: 347–361.
Petrelli A, Circosta P, Granziero L, Mazzone M, Pisacane A, Fenoglio S et al. (2006). Ab-induced ectodomain shedding mediates hepatocyte growth factor receptor down-regulation and hampers biological activity. Proc Natl Acad Sci USA 103: 5090–5095.
Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S et al. (1994). A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell 77: 261–271.
Pouyssegur J, Dayan F, Mazure NM . (2006). Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 441: 437–443.
Prat M, Crepaldi T, Gandino L, Giordano S, Longati P, Comoglio P . (1991). C-terminal truncated forms of Met, the hepatocyte growth factor receptor. Mol Cell Biol 11: 5954–5962.
Rege-Cambrin G, Scaravaglio P, Carozzi F, Giordano S, Ponzetto C, Comoglio PM et al. (1992). Karyotypic analysis of gastric carcinoma cell lines carrying an amplified c-met oncogene. Cancer Genet Cytogenet 64: 170–173.
Rong S, Segal S, Anver M, Resau JH, Vande Woude GF . (1994). Invasiveness and metastasis of NIH 3T3 cells induced by Met-hepatocyte growth factor/scatter factor autocrine stimulation. Proc Natl Acad Sci USA 91: 4731–4735.
Rosario M, Birchmeier W . (2004). Making tubes: step by step. Dev Cell 7: 3–5.
Schmidt L, Junker K, Nakaigawa N, Kinjerski T, Weirich G, Miller M et al. (1999). Novel mutations of the MET proto-oncogene in papillary renal carcinomas. Oncogene 18: 2343–2350.
Shinomiya N, Gao CF, Xie Q, Gustafson M, Waters DJ, Zhang YW et al. (2004). RNA interference reveals that ligand-independent met activity is required for tumor cell signaling and survival. Cancer Res 64: 7962–7970.
Skibinski G, Skibinska A, James K . (2001). The role of hepatocyte growth factor and its receptor c-met in interactions between lymphocytes and stromal cells in secondary human lymphoid organs. Immunology 102: 506–514.
Smolen GA, Sordella R, Muir B, Mohapatra G, Barmettler A, Archibald H et al. (2006). Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc Natl Acad Sci USA 103: 2316–2321.
Takayama H, Larochelle WJ, Sharp R, Otsuka T, Kriebel P, Anver M et al. (1997). Diverse tumorigenesis associated with aberrant development in mice overexpressing hepatocyte growth factor/scatter factor. Proc Natl Acad Sci USA 94: 701–706.
Taulli R, Accornero P, Follenzi A, Mangano T, Morotti A, Scuoppo C et al. (2005). RNAi technology and lentiviral delivery as a powerful tool to suppress Tpr-Met-mediated tumorigenesis. Cancer Gene Ther 12: 456–463.
Taulli R, Scuoppo C, Bersani F, Accornero P, Forni PE, Miretti S et al. (2006). Validation of met as a therapeutic target in alveolar and embryonal rhabdomyosarcoma. Cancer Res 66: 4742–4749.
Thiery JP . (2003). Epithelial–mesenchymal transitions in development and pathologies. Curr Opin Cell Biol 15: 740–746.
Thompson EW, Newgreen DF, Tarin D . (2005). Carcinoma invasion and metastasis: a role for epithelial–mesenchymal transition? Cancer Res 65: 5991–5995.
Trusolino L, Comoglio PM . (2002). Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat Rev Cancer 2: 289–300.
Trusolino L, Bertotti A, Comoglio PM . (2001). A signaling adapter function for alpha6beta4 integrin in the control of HGF-dependent invasive growth. Cell 107: 643–654.
Valabrega G, Montemurro F, Sarotto I, Petrelli A, Rubini P, Tacchetti C et al. (2005). TGFalpha expression impairs Trastuzumab-induced HER2 downregulation. Oncogene 24: 3002–3010.
van de Wetering M, Oving I, Muncan V, Pon Fong MT, Brantjes H, van Leenen D et al. (2003). Specific inhibition of gene expression using a stably integrated, inducible small-interfering-RNA vector. EMBO R 4: 609–615.
Vande Woude GF, Jeffers M, Cortner J, Alvord G, Tsarfaty I, Resau J . (1997). Met-HGF/SF: tumorigenesis, invasion and metastasis. Ciba Found Symp 212: 119–130.
Vigna E, Naldini L . (2000). Lentiviral vectors: excellent tools for experimental gene transfer and promising candidates for gene therapy. J Gene Med 2: 308–316.
Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L et al. (2002). Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 20: 719–726.
Wang R, Ferrell LD, Faouzi S, Maher JJ, Bishop JM . (2001). Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J Cell Biol 153: 1023–1034.
Zhang YW, Vande Woude GF . (2003). HGF/SF-met signaling in the control of branching morphogenesis and invasion. J Cell Biochem 88: 408–417.
Acknowledgements
We thank E Vigna and L Naldini for providing lentiviral vectors; L Tamagnone and our colleagues for helpful discussions; R Albano, R LoNoce and L Palmas for technical assistance. This study was supported by AIRC (grants to SG and PMC), MIUR (grant to SG) and Regione Piemonte (grant to SG). CM is the recipient of a Lagrange fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Rights and permissions
About this article
Cite this article
Corso, S., Migliore, C., Ghiso, E. et al. Silencing the MET oncogene leads to regression of experimental tumors and metastases. Oncogene 27, 684–693 (2008). https://doi.org/10.1038/sj.onc.1210697
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210697
Keywords
This article is cited by
-
Decoding cancer insights: recent progress and strategies in proteomics for biomarker discovery
Journal of Proteins and Proteomics (2024)
-
Mass spectrometry-based proteomics as an emerging tool in clinical laboratories
Clinical Proteomics (2023)
-
Known and novel roles of the MET oncogene in cancer: a coherent approach to targeted therapy
Nature Reviews Cancer (2018)
-
Dual MET/EGFR therapy leads to complete response and resistance prevention in a MET-amplified gastroesophageal xenopatient cohort
Oncogene (2017)
-
Medullary Thyroid Cancer: Clinical Characteristics and New Insights into Therapeutic Strategies Targeting Tyrosine Kinases
Molecular Diagnosis & Therapy (2017)