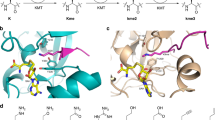
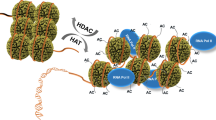
Abstract
The post-translational modification of histones plays an important role in chromatin regulation, a process that insures the fidelity of gene expression and other DNA transactions. Of the enzymes that mediate post-translation modification, the histone acetyltransferase (HAT) and histone deacetylase (HDAC) proteins that add and remove acetyl groups to and from target lysine residues within histones, respectively, have been the most extensively studied at both the functional and structural levels. Not surprisingly, the aberrant activity of several of these enzymes have been implicated in human diseases such as cancer and metabolic disorders, thus making them important drug targets. Significant mechanistic insights into the function of HATs and HDACs have come from the X-ray crystal structures of these enzymes both alone and in liganded complexes, along with associated enzymatic and biochemical studies. In this review, we will discuss what we have learned from the structures and related biochemistry of HATs and HDACs and the implications of these findings for the design of protein effectors to regulate gene expression and treat disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Anekonda TS, Reddy PH . (2006). Neuronal protection by sirtuins in Alzheimer's disease. J Neurochem 96: 305–313.
Avalos JL, Bever KM, Wolberger C . (2005). Mechanism of sirtuin inhibition by nicotinamide: altering the NAD(+) cosubstrate specificity of a Sir2 enzyme. Mol Cell 17: 855–868.
Avalos JL, Boeke JD, Wolberger C . (2004). Structural basis for the mechanism and regulation of Sir2 enzymes. Mol Cell 13: 639–648.
Balasubramanyam K, Altaf M, Varier RA, Swaminathan V, Ravindran A, Sadhale PP et al. (2004a). Polyisoprenylated benzophenone, garcinol, a natural histone acetyltransferase inhibitor, represses chromatin transcription and alters global gene expression. J Biol Chem 279: 33716–33726.
Balasubramanyam K, Varier RA, Altaf M, Swaminathan V, Siddappa NB, Ranga U et al. (2004b). Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J Biol Chem 279: 51163–51171.
Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A et al. (2006). Resveratrol improves health and survival of mice on a high-calorie diet [see comment]. Nature 444: 337–342.
Berndsen CE, Albaugh BN, Tan S, Denu JM . (2007). Catalytic mechanism of a MYST family histone acetyltransferase. Biochemistry 46: 623–629.
Biel M, Wascholowski V, Giannis A . (2005). Epigenetics – an epicenter of gene regulation: histones and histone-modifying enzymes. Angew Chem Int Ed Engl 44: 3186–3216.
Bolden JE, Peart MJ, Johnstone RW . (2006). Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5: 769–784.
Borra MT, Smith BC, Denu JM . (2005). Mechanism of human SIRT1 activation by resveratrol. J Biol Chem 280: 17187–17195.
Brownell J, Allis C . (1996). Special HATs for special occasions: linking histone acetylation to chromatin assembly and gene activation. Curr Opin Genet Dev 6: 176–184.
Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y et al. (2004). Stress-dependant regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303: 2011–2015.
Chaffanet M, Gressin L, Preudhomme C, Soenen-Cornu V, Birnbaum D, Pebusque MJ . (2000). MOZ is fused to p300 in an acute monocytic leukemia with t(8;22). Genes Chromosomes Cancer 28: 138–144.
Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P et al. (2003). Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci USA 100: 10794–10799.
Clements A, Marmorstein R . (2003). Structure and function of histone acetyltransferase domains. Methods Enzymol 371: 545–564.
Clements A, Rojas JR, Trievel RC, Wang L, Berger SL, Marmorstein R . (1999). Crystal structure of the histone acetyltransferase domain of the human P/CAF transcriptional regulator bound to coenzyme-A. EMBO J 18: 3521–3532.
de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB . (2003). Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J 370: 737–749.
Finnin MS, Donigian JR, Cohen A, Richon VM, Rifkind RA, Marks PA et al. (1999). Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature 401: 188–193.
Frye RA . (2000). Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun 273: 793–798.
Gallinari P, Di Marco S, Jones P, Pallaoro M, Steinkuhler C . (2007). HDACs, histone deacetylation and gene transcription: from molecular biology to cancer therapuetics. Cell Res 17: 195–211.
Giles RH, Peters DJM, Breuning MH . (1998). Conjunction dysfunction: CBP/p300 in human disease. Trends Genet 14: 178–183.
Glozak MA, Sengupta N, Zhang X, Seto E . (2005). Acetylation and deacetylation of non-histone proteins. Gene 363: 15–23.
Gregoretti IV, Lee YM, Goodson HV . (2004). Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol 338: 17–31.
Haigis MC, Guarente LP . (2006). Mammalian sirtuins – emerging roles in physiology, aging, and calorie restriction. Genes Develop 20: 2913–2921.
Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ et al. (2006). SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell 126: 941–954.
Heltweg B, Gatbonton T, Schuler AD, Posakony J, Li H, Goehle S et al. (2006). Antitumor activity of a small-molecule inhibitor of human silent information regulator 2 enzymes. Cancer Res 66: 4368–4377.
Hideshima T, Bradner JE, Wong J, Chauhan D, Richardson P, Schreiber SL et al. (2005). Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc Natl Acad Sci USA 102: 8567–8572.
Hoff KG, Avalos JL, Sens K, Wolberger C . (2006). Insights into the sirtuin mechanism from ternary complexes containing NAD+ and acetylated peptide. Structure 14: 1231–1240.
Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG et al. (2003). Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425: 191–196.
Imai S, Johnson FB, Marciniak RA, McVey M, Park PU, Guarente L . (2000). Sir2: an NAD-dependent histone deacetylase that connects chromatin silencing, metabolism, and aging. Cold Spring Harb Symp Quant Biol 65: 297–302.
Jackson MD, Denu JM . (2002). Structural identification of 2′- and 3′-O-acetyl-ADP-ribose as novel metabolites derived from the Sir2 family of beta-NAD+-dependent histone/protein deacetylases. J Biol Chem 277: 18535–18544.
Jackson MD, Schmidt MT, Oppenheimer NJ, Denu JM . (2003). Mechanism of nicotinamide inhibition and transglycosidation by Sir2 histone/protein deacetylases. J Biol Chem 278: 50985–50998.
Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD et al. (2005). Substrate-specific activation of sirtuins by resveratrol. J Biol Chem 280: 17038–17045.
Kelly WK, O’Connor OA, Marks PA . (2002). Histone deacetylase inhibitors: from target to clinical trials. Expert Opin Investig Drugs 11: 1695–1713.
Kelly WK, Richon VM, O’Connor O, Curley T, MacGregor-Curtelli B, Tong W et al. (2003). Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. Clin Cancer Res 9: 3578–3588.
Khan AN, Lewis PN . (2006). Use of substrate analogs and mutagenesis to study substrate binding and catalysis in the Sir2 family of NAD-dependent protein deacetylases. J Biol Chem 281: 11702–11711.
Kiviranta PH, Leppanen J, Kyrylenko S, Salo HS, Lahtela-Kakkonen M, Tervo AJ et al. (2006). N,N′-bisbenzylidenebenzene-1,4-diamines and N,N′-bisbenzylidenenaphthalene-1,4-diamines as Sirtuin type 2 (SIRT2) inhibitors. J Med Chem 49: 7907–7911.
Kobayashi Y, Furukawa-Hibi Y, Chen C, Horio Y, Isobe K, Ikeda K et al. (2005). SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int J Mol Med 16: 237–243.
Kumagai T, Wakimoto N, Yin D, Gery S, Kawamata N, Takai N et al. (2007). Histone deacetylase inhibitor, suberoylanilide hydroxamic acid (Vorinostat, SAHA) profoundly inhibits the growth of human pancreatic cancer cells. Int J Cancer 121: 656–665.
Lau OD, Courtney AD, Vassilev A, Marzilli LA, Cotter RJ, Nakatani Y et al. (2000a). p300/CBP-associated factor histone acetyltransferase processing of a peptide substrate. Kinetic analysis of the catalytic mechanism. J Biol Chem 275: 21953–21959.
Lau OD, Kundu TK, Soccio RE, Ait-Si-Ali S, Khalil EM, Vassilev A et al. (2000b). HATs off: selective synthetic inhibitors of the histone acetyltransferases p300 and PCAF. Mol Cell 5: 539–595.
Lin HY, Chen CS, Lin SP, Weng JR, Chen CS . (2006). Targeting histone deacetylase in cancer therapy. Med Res Rev 26: 397–413.
Lin Y, Fletcher CM, Zhou J, Allis CD, Wagner G . (1999). Solution structure of the catalytic domain of Tetrahymena GCN5 histone acetyltransferase in complex with coenzyme A. Nature 400: 86–89.
Liszt G, Ford E, Kurtev M, Guarente L . (2005). Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J Biol Chem 280: 21313–21320.
Loyola A, Almouzni G . (2004). Histone chaperones, a supporting role in the limelight. Biochim Biophys Acta 1677: 3–11.
Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ . (1997). Crystal structure of the nucleosome core particle at 2.8 angstrom resolution. Nature 389: 251–260.
Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A et al. (2001). Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 107: 137–148.
Manson MM, Farmer PB, Gescher A, Steward WP . (2005). Innovative agents in cancer prevention. Recent Results Cancer Res 166: 257–275.
Marks PA, Jiang X . (2005). Histone deacetylase inhibitors in programmed cell death and cancer therapy. Cell Cycle 4: 549–551.
Marmorstein R . (2001a). Structure and function of histone acetyltransferases. Cell Mol Life Sci 58: 693–703.
Marmorstein R . (2001b). Structure of histone deacetylases: insights into substrate recognition and catalysis. Structure 9: 1127–1133.
Marmorstein R . (2004). Structure and chemistry of the Sir2 family of NAD+-dependent histone/protein deactylases. Biochem Soc Trans 32: 904–909.
Marmorstein R, Roth SY . (2001a). Histone acetyltransferases: function, structure, and catalysis. Curr Opin Genet Dev 11: 155–161.
Marmorstein R, Roth SY . (2001b). Histone acetyltransferases: function, structure, and catalysis. Curr Opin Genet Dev 11: 155–161.
Mizzen CA, Allis CD . (1998). Linking histone acetylation to transcriptional regulation. Cell Mol Life Sci 54: 6–20.
Moreth K, Riester D, Hildmann C, Hempel R, Wegener D, Schober A et al. (2007). An active site tyrosine residue is essential for amidohydrolase but not for esterase activity of a class 2 histone deacetylase-like bacterial enzyme. Biochem J 401: 659–665.
Muraoka M, Konishi M, KikuchiYanoshita R, Tanaka K, Shitara N, Chong JM et al. (1996). p300 gene alterations in colorectal and gastric carcinomas. Oncogene 12: 1565–1569.
Neuwald AF, Landsman D . (1997). GCN5-related histone N-acetyltransferases belong to a diverse superfamily that include the yeast SPT10 protein. Trends Biochem Sci 22: 154–155.
Nielsen TK, Hildmann C, Dickmanns A, Schwienhorst A, Ficner R . (2005). Crystal structure of a bacterial class 2 histone deacetylase homologue. J Mol Biol 354: 107–120.
Nielsen TK, Hildmann C, Riester D, Wegener D, Schwienhorst A, Ficner R . (2007). Complex structure of a bacterial class 2 histone deacetylase homologue with a trifluromethylketone inhibitor. Acta Crystallogr Sect F Struc Biol Cryst Commun 63: 270–273.
Nightingale KP, O’Neill L P, Turner BM . (2006). Histone modifications: signalling receptors and potential elements of a heritable epigenetic code. Curr Opin Genet Dev 16: 125–136.
Parker JA, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H et al. (2005). Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet 37: 349–350.
Parthun MR et al. (2007) Hat1: the emerging cellular roles of a type ‘B’ histone acetyltransferase. Oncogene 26: 5319–5328.
Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Oliveira RM et al. (2004). Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 429: 771–776.
Posakony J, Hirao M, Stevens S, Simon JA, Bedalov A . (2004). Inhibitors of Sir2: evaluation of splitomicin analogues. J Med Chem 47: 2635–2644.
Poux AN, Marmorstein R . (2003). Molecular basis for Gcn5/PCAF histone acetyltransferase selectivity for histone and nonhistone substrates. Biochemistry 42: 14366–14374.
Poux AN, Cebrat M, Kim CM, Cole PA, Marmorstein R . (2002). Structure of the GCN5 histone acetyltransferase bound to a bisubstrate inhibitor. Proc Natl Acad Sci USA 99: 14065–14070.
Rojas JR, Trievel RC, Zhou J, Mo Y, Li X, Berger SL et al. (1999). Structure of the Tetrahymena GCN5 bound to coenzyme-A and a histone H3 peptide. Nature 401: 93–98.
Sagar V, Zheng W, Thompson PR, Cole PA . (2004). Bisubstrate analogue structure–activity relationships for p300 histone acetyltransferase inhibitors. Bioorg Med Chem 12: 3383–3390.
Sanders BD, Zhao K, Slama JT, Marmorstein R . (2007). Structural basis for nicotinamide inhibition and base exchange in sir2 enzymes. Mol Cell 25: 463–472.
Sauve AA, Schramm VL . (2003). Sir2 regulation by nicotinamide results from switching between base exchange and deacetylation chemistry. Biochemistry 42: 9249–9256.
Sauve AA, Celic I, Avalos J, Deng H, Boeke JD, Schramm VL . (2001a). Chemistry of gene silencing: the mechanism of NAD+-dependent deacetylation reactions. Biochemistry 40: 15456–15463.
Sauve AA, Celic I, Avalos J, Deng H, Boeke JD, Schramm VL . (2001b). Chemistry of gene silencing: the mechanism of NAD+-dependent deacetylation reactions. Biochemistry 40: 15456–15463.
Sauve AA, Wolberger C, Schramm VL, Boeke JD . (2006). The biochemistry of sirtuins. Annu Rev Biochem 75: 435–465.
Schuetz A, Min J, Antoshenko T, Wang CL, Allali-Hassani A, Dong A et al. (2007). Structural basis of inhibition of the human NAD+-dependent deacetylase SIRT5 by suramin. Structure 15: 377–389.
Serrador JM, Cabrero JR, Sancho D, Mittelbrunn M, Urzainqui A, Sanchez-Madrid F . (2004). HDAC6 deacetylase activity links the tubulin cytoskeleton with immune synapse organization. Immunity 20: 417–428.
Smith BC, Denu JM . (2007). Sir2 deacetylases exhibit nucleophilic participation of acetyl-lysine in NAD+ cleavage. J Am Chem Soc 129: 5802–5805.
Somoza JR, Kene RJ, Katz BA, Mol C, Ho JD, Jennings AJ : et al. (2004). Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure 12: 1325–1334.
Sterner DE, Berger SL . (2000a). Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev 64: 435–459.
Sterner DE, Berger SL . (2000b). Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev 64: 435–459.
Saunders LR, Verdin EL . (2007) Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene 26: 5489–5504.
Tanner KG, Trievel RC, Kuo MH, Howard RM, Berger SL, Allis CD et al. (1999). Catalytic mechanism and function of invariant glutamic acid 173 from the histone acetyltransferase GCN5 transcriptional coactivator. J Biol Chem 274: 18157–18160.
Thompson PR, Wang D, Wang L, Fulco M, Pediconi N, Zhang D et al. (2004). Regulation of the p300 HAT domain via a novel activation loop. Nat Struct Mol Biol 11: 308–315.
Timmermann S . (2001). Histone acetylation and disease. Cell Mol Life Sci 58: 728–736.
Trievel RC, Rojas JR, Sterner DE, Venkataramani R, Wang L, Zhou J et al. (1999). Crystal structure and mechanism of histone acetylation of the yeast GCN5 transcriptional coactivator. Proc Natl Acad Sci USA 96: 8931–8936.
Vannini A, Volpari C, Filocamo G, Casavola E, Brunetti M, Rezoni D et al. (2004). Crystal structure of a eukaryotic zinc-dependent histone deacetylase, human HDAC8, complexed with a hydroxamic acid inhibitor. Proc Natl Acad Sci USA 101: 15064–15069.
Vanommeslaeghe K, De Proft F, Loverix S, Tourwe D, Geerlings P . (2005). Theoretical study revealing the functioning of a novel combination of catalytic motifs in histone deacetylase. Bioorg Med Chem 13: 3987–3992.
Varga-Weisz PD, Becker PB . (2006). Regulation of higher-order chromatin structures by nucleosome-remodelling factors. Curr Opin Genet Dev 16: 151–156.
Varier RA, Swaminathan V, Balasubramanyam K, Kundu TK . (2004). Implications of small molecule activators and inhibitors of histone acetyltransferases in chromatin therapy. Biochem Pharmacol 68: 1215–1220.
Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M et al. (2004a). Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430: 686–689.
Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M et al. (2004b). Sirtuin activators mimic caloric restriction and delay ageing in metazoans [erratum appears in Nature 2004; 431: 107]. Nature 430: 686–689.
Woodcock CL . (2006). Chromatin architecture. Curr Opin Struct Biol 16: 213–220.
Yan Y, Barlev NA, Haley RH, Berger SL, Marmorstein R . (2000). Crystal structure of yeast Esa1 suggests a unified mechanism of catalysis and substrate binding by histone acetyltransferases. Mol Cell 6: 1195–1205.
Yan Y, Harper S, Speicher DW, Marmorstein R . (2002). The catalytic mechanism of the ESA1 histone acetyltransferase involves a self-acetylated intermediate. Nat Struct Biol 9: 862–869.
Zhang K, Dent SY . (2005). Histone modifying enzymes and cancer: going beyond histones. J Cell Biochem 96: 1137–1148.
Zhao K, Harshaw R, Chai X, Marmorstein R . (2004). Structural basis for nicotinamide cleavage and ADP-ribose transfer by NAD+-dependent Sir2 histone/protein deacetylases. Proc Natl Acad Sci USA 101: 8563–8568.
Zheng Y, Thompson PR, Cebrat M, Wang L, Devlin MK, Alani RM et al. (2004). Selective HAT inhibitors as mechanistic tools for protein acetylation. Methods Enzymol 376: 188–199.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hodawadekar, S., Marmorstein, R. Chemistry of acetyl transfer by histone modifying enzymes: structure, mechanism and implications for effector design. Oncogene 26, 5528–5540 (2007). https://doi.org/10.1038/sj.onc.1210619
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210619
Keywords
This article is cited by
-
Epigenetics as a versatile regulator of fibrosis
Journal of Translational Medicine (2023)
-
Epigenetic regulation in the tumor microenvironment: molecular mechanisms and therapeutic targets
Signal Transduction and Targeted Therapy (2023)
-
Evaluation of post-translational modifications in histone proteins: A review on histone modification defects in developmental and neurological disorders
Journal of Biosciences (2020)
-
Natural products development under epigenetic modulation in fungi
Phytochemistry Reviews (2020)
-
Epigenetics in ovarian cancer: premise, properties, and perspectives
Molecular Cancer (2018)