Abstract
Initiation of smoking behavior typically occurs during adolescence and rarely occurs during adulthood. Despite this epidemiological evidence, relatively little is known about possible neurobiological differences in the response to nicotine in adolescents that might make them more vulnerable to nicotine addiction. In the current study, we assessed nicotine self-administration under fixed ratio (FR) and progressive ratio (PR) reinforcement schedules in adolescent (postnatal day (P) 33–35) and adult (P91–94) rats. We then assessed extinction and reinstatement of nicotine seeking in adulthood in rats that initiated nicotine self-administration during either adolescence or adulthood. Nicotine self-administration (0.03 mg/kg/infusion, i.v.) was higher in adult rats than in adolescent rats under FR5 and PR reinforcement schedules; no age differences in nicotine self-administration were observed under FR1 or FR2 reinforcement schedules. In contrast, saccharin self-administration under FR5 and PR reinforcement schedules was similar in both age groups, potentially ruling out age differences in general performance. Rats that initiated nicotine self-administration as adults demonstrated a greater resistance to extinction of nicotine taking behavior when saline was substituted for nicotine than rats that initiated self-administration as adolescents. Reinstatement of nicotine seeking following nicotine priming injections (0.075, 0.15, 0.3 mg/kg, s.c.) was independent of the age of onset of nicotine self-administration. The present data from established rat models of drug self-administration and drug relapse suggest that nicotine is less reinforcing in adolescent compared with adult rats and that processes other than the reinforcing effects of nicotine may be involved in the greater susceptibility to smoking during the adolescent developmental stage.
Similar content being viewed by others
INTRODUCTION
Initiation of tobacco use typically occurs during adolescence, with 80% of adult smokers reporting they first used tobacco prior to age 18 (DeWit et al, 1997; Clark et al, 1998; Eissenberg and Balster, 2000). Early exposure to tobacco has long-term adverse consequences: the progression from tobacco use to the use of other illicit drugs is more rapid when onset of use occurs during adolescence (Kandel et al, 1992; Yu and Williford, 1992), and early smoking onset is associated with a reduced probability of quitting (Breslau and Peterson, 1996; Chen and Millar, 1998).
Research into factors contributing to early tobacco use has shown that psychosocial factors, such as peer and family influences (Simons-Morton et al, 2001), and behavioral characteristics associated with adolescence, including elevated sensation seeking and risk taking (Arnett, 1992; Coogan et al, 1998), play important roles in the initiation of cigarette smoking. Another possibility for the greater susceptibility to smoking in adolescents is increased sensitivity to the rewarding effects of nicotine during this developmental phase. However, due to methodological and ethical concerns, this issue cannot be assessed in humans and requires the use of animal models.
The adolescent period in rodents is typically estimated to span postnatal (P) days 28–42 (Spear and Brake, 1983); however, other estimates have extended the age range to include up to postnatal day 55 (Spear, 2000; Chen et al, 2007; Frantz et al, 2007). Adolescent rats share many behavioral and neurobiological characteristics with human adolescents, and have been useful in determining factors contributing to vulnerability to drugs, including nicotine, during this ontogenetic period (Spear, 2000). Using conditioned place preference (CPP) and conditioned taste avoidance (CTA) procedures, we and others reported that adolescent rats are more sensitive to the rewarding effects of nicotine and less sensitive to its aversive effects than adult rats (Vastola et al, 2002; Belluzzi et al, 2004; Torrella et al, 2004; Wilmouth and Spear, 2004; Shram et al, 2006). Furthermore, several investigators reported that adolescent rats may acquire nicotine self-administration faster than adult rats (Levin et al, 2003; Belluzzi et al, 2005; Chen et al, 2007). However, the degree to which the findings from these self-administration studies reflect increased vulnerability to nicotine's rewarding effects in adolescents is unknown.
In the current series of experiments, we examined nicotine self-administration in adolescent and adult rats under low-response cost fixed ratio (FR) reinforcement schedules and a progressive ratio (PR) schedule. The PR schedule is regarded as a valid procedure for assessing the reinforcing efficacy of drugs of abuse (Richardson and Roberts, 1996; Stafford et al, 1998), and it has been used to assess nicotine's reinforcing efficacy in laboratory animals and humans (Risner and Goldberg, 1983; Donny et al, 1999; Harvey et al, 2004). Since early onset of tobacco use is associated with a higher probability of relapse during abstinence (Cui et al, 2006), a further objective was to assess the reinstatement of nicotine seeking induced by acute re-exposure to the drug (nicotine priming) in nicotine-free adult rats that were trained to self-administer nicotine either during adolescence or adulthood using the reinstatement model, an animal model of relapse to drug seeking (Shaham et al, 2003; Epstein et al, 2006). Finally, we examined age differences in saccharin self-administration to determine the specificity of our findings with nicotine.
MATERIALS AND METHODS
Subjects
Fifty-eight 53–56-day-old male Long Evans rats and 18 pregnant Long Evans dams were purchased from Charles River Laboratories (QC, Canada). Adult rats were group housed (n=4 per cage), and dams were singly housed in Plexiglas cages (51 × 41 × 20 cm). Pregnant dams were used as the source for adolescent rats instead of purchasing 21-day-old rats in order to avoid transport stress to juveniles, which would have to undergo acclimatization, food training and surgery within 10 days of arrival such that testing could begin during adolescence. Fifty-eight male pups were weaned and housed by litter on postnatal day 20 (P20). Rats were maintained on a 12/12 h light/dark cycle (lights on at 1900) in a humidity- and temperature-regulated vivarium. Water and Purina rat chow were available ad libitum until the training phase of each experiment. Subsequently, rats were fed 20–25 g of rat chow per day following their daily operant session.
Apparatus
Nicotine self-administration occurred in eight operant chambers operated by a computer-controlled interface system (Med Associates, St Albans, VT). Each chamber was equipped with two levers located 2.5 cm above a removable grid floor. Depressing the active lever activated a high-speed microliter syringe pump (PHM-104, Med Associates). Pressing the inactive lever was recorded, but had no programmed consequences. A white cue light was positioned 7.5 cm above the active lever, and a tone generator (2900 Hz) was located directly above the cue light; both visual (40 s) and auditory (1 s) stimuli were turned on when the active lever was pressed. A houselight was located on the opposite side of the chamber and signaled the onset of the self-administration session. A modified 22-gauge cannula, which was attached to the intravenous catheter on a daily basis, was connected to a fluid swivel with Tygon tubing protected by a metal spring. The swivel was attached to the Hamilton syringe with Tygon tubing.
Saccharin self-administration occurred in eight similarly equipped operant chambers (Med Associates), with the exception of a liquid drop receptacle located between the active and inactive levers. Responding on the active lever resulted in the activation of the visual (6 s) and auditory (1 s) stimuli and a syringe pump (PHM-100, Med Associates) equipped with a 60 ml syringe, which delivered 0.1 ml saccharin over 6 s.
Surgery
Rats were anesthetized using a ketamine/xylazine mixture (75 mg/kg ketamine/10 mg/kg xylazine; 2 ml/kg, i.p.). Incision sites were treated with a local anesthetic (0.1 ml Marcaine 0.125%, s.c.). Buprenorphine (0.01 mg/kg, s.c.) was administered as an analgesic and penlong (15 000 (juvenile) or 30 000 U (adult), i.m., Rogar/STP, London, ON, Canada) was used as antibiotic treatment. Juvenile (P26–28) and adult rats (P80–87) were prepared with catheters implanted into the right jugular vein as described previously (Corrigall and Coen, 1989; Le et al, 2006). The catheter exited between the scapulae and was attached to the modified 22-gauge cannula that connected to the fluid swivel system. The rats, now individually housed, were allowed to recover from surgery for 6–8 days. Catheters were flushed daily with 0.1 ml of a sterile heparin-saline solution (50 U/ml) to maintain patency.
Drugs
Nicotine solutions (Sigma-Aldrich, Oakville, ON, Canada) were prepared daily using sterile saline, and pH was adjusted to 6.8–7.2. The unit doses for nicotine self-administration were 0.015, 0.03 and 0.06 mg/kg/infusion, expressed as base (Corrigall and Coen, 1989; Shoaib and Stolerman, 1999; Le et al, 2006). During the tests for reinstatement, nicotine (0.075, 0.15, 0.3 mg/kg) was administered subcutaneously in a volume of 1 ml/kg (Shaham et al, 1997; Le et al, 2006). Catheter patency was tested after each experimental phase using the rapid acting anesthetic, sodium methohexital (0.05 mg/kg, i.v., 10 mg/ml).
Procedures
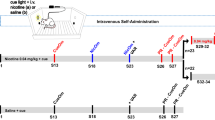
Experiment 1: nicotine self-administration and dose-response
Before surgery, 20 juvenile (P21–25) and 20 adult (P75–79) rats underwent operant training for 45 mg sucrose pellets (Bioserv, Frenchtown, NJ) at an FR1 reinforcement schedule in operant chambers equipped with pellet magazines. Rats could earn up to 400 pellets during each of two 8-h training sessions conducted over 2 consecutive days; water was available at all times. One juvenile rat failed to learn to how to lever press and was excluded from the study.
Once the younger animals reached adolescence, both adolescent (P34–35) and adult (P91–94) rats initiated self-administration of nicotine (0.03 mg/kg/infusion) under an FR1 schedule for six daily 1-h sessions. Timeout following nicotine infusion was 40 s and pressing on the active lever had no programmed consequence but was recorded. Rats were then placed under FR2 and FR5 schedules for three sessions each. Subsequently, a nicotine dose–response curve was determined when adolescents were P46–56 and adults were P103–113. Doses were presented in the following order: 0.03, 0.015, and 0.06 mg/kg/infusion. Each rat had three sessions at each infusion dose. Following the nicotine dose–response curve, rats received four sessions at the 0.03 mg/kg/infusion training dose. Rats were then allowed to respond for this dose on a PR schedule that has previously been reported by others (Depoortere et al, 1993; Donny et al, 1999); the sequence was determined using the exponential formula (5 exp(0.2 × infusion number)−5), such that the required responses per infusion are as follows: 3, 6, 10, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 179, 219, 268, 328, 402, 492, 603. PR conditions were the same as in the FR sessions, with the exception that sessions were 2 h in duration. Breakpoint was achieved when >20 min of inactivity on the active lever elapsed.
Experiment 2: nicotine self-administration, extinction, and reinstatement
Experiment 2 was conducted because the younger rats (P60–62) in Experiment 1 were no longer adolescents by the time of PR testing.
Before surgery, juvenile (n=18) and adult (n=17) rats underwent operant training as described in Experiment 1. Once the younger rats reached adolescence, both adolescent (P33–34) and adult (P94–97) rats initiated self-administration of nicotine (0.03 mg/kg/infusion) under an FR1 schedule for six sessions and under FR2 for four sessions.
PR testing
PR testing was conducted over four sessions when adolescents were P42–45 and adults were P103–106. Following PR testing, rats were placed under an FR5 schedule for 4 days before extinction sessions.
Extinction
Extinction conditions were the same as the nicotine self-administration sessions with the exception that pressing on the active lever resulted in the infusion of saline instead of nicotine. Rats were given 10–34 extinction sessions until they achieved extinction criterion of less than 15 lever presses on the active lever in two consecutive extinction sessions.
Reinstatement
The rats were administered s.c. saline injections (two sessions) as a baseline for nicotine priming-induced reinstatement. Following habituation to saline administration, priming injections of nicotine (0.075, 0.15, and 0.3 mg/kg, s.c.) were administered 30 min before testing for responding on the nicotine-associated lever in a counterbalanced order, and with a minimum of one session between priming sessions, or until extinction criterion was again attained.
Experiment 3: saccharin self-administration, dose–response, extinction
Following operant training, adolescent (P33–34, n=14) and adult (P94–99, n=14) rats initiated self-administration of saccharin (0.05%, dissolved in tap water) under an FR1 schedule for six daily 1-h sessions. Timeout following saccharin delivery was 6 s and pressing on the active lever had no programmed consequence but was recorded. Rats were then placed under FR2 and FR5 schedules for three sessions each. Subsequently, a saccharin concentration–response curve was determined when adolescents were P45–60 and adults were P106–121. Saccharin concentrations were presented in the following order: 0.05, 0.10, 0.20, and 0.025%. Rats had three sessions at each concentration. Following the concentration–response curve, rats received two sessions at the 0.1% concentration. Rats that initiated saccharin self-administration as adolescents or as adults (P62–64 and P123–125, respectively) were then allowed to respond for this concentration under the same PR conditions as in the nicotine experiments; this concentration produced levels of responding comparable to that of rats trained to self-administer nicotine under FR5 conditions. Following PR testing, the rats were placed under an FR5 schedule for 5 days before extinction sessions.
Extinction conditions were the same as those during the saccharin self-administration sessions with the exception that no saccharin was delivered upon responding on the active lever. Rats were given 14–38 extinction sessions until they achieved extinction criterion of less than 15 lever presses on the active lever in two consecutive extinction sessions.
Experiment 4: saccharin self-administration under a PR schedule
Experiment 4 was conducted because the younger rats (P62–64) in Experiment 3 were no longer adolescents by the time of PR testing. Following operant training, adolescent (P33, n=8) and adult (P97–100, n=8) rats initiated self-administration of saccharin (0.2%) under an FR1 schedule for four sessions and under an FR2 schedule for three sessions; the concentration was increased to 0.2% in an attempt to increase the breakpoints for saccharin. The PR testing was conducted as described above over two sessions when adolescents were P40–41 and adults were P104–108.
Statistical Analysis
Data were analyzed using analyses of variance (ANOVAs) with the between-subjects factor of age. Different phases were analyzed separately and included all rats with patent catheters up until that point. The nonparametric median test was used to analyze age differences in breakpoint, or last completed ratio, to avoid violating the assumption of homogeneity of variance (Richardson and Roberts, 1996). Significance was set at α=0.05. Tukey's honestly significant difference (HSD) was employed for all post hoc tests where appropriate.
RESULTS
Experiment 1: Nicotine Self-Administration and Dose–Response
Figure 1a shows mean (±SEM) number of nicotine infusions during the first 13 self-administration sessions under the FR1, FR2, and FR5 reinforcement schedules. Under the FR1 and FR2 schedules, adolescent and adult nicotine intake was similar. During the FR1 sessions, adolescent and adult rats earned a similar number of nicotine infusions (p>0.05), and this remained stable across sessions (p>0.05) and did not vary with age (p>0.05). Nicotine infusions earned remained similar when the response requirement increased to FR2, and no age differences were detected (p>0.05). Number of infusions earned increased across FR2 sessions (p>0.05), independent of age (p>0.05).
Nicotine self-administration in adolescent and adult rats during Experiment 1. (a) Mean±SEM number of nicotine infusions earned during the first 13 sessions of nicotine self-administration (0.03 mg/kg/infusion), under FR1, FR2, and FR5 schedules of reinforcement. (b) Mean±SEM number of nicotine infusions earned during the dose–response determination; data are an average from three sessions at each dose; n=10–14 per age. (c) Bars represent mean±SEM number of nicotine infusions earned by rats that initiated nicotine self-administration as adolescents (adolescent-onset) and adults during three 2 h PR sessions; lines represent median breakpoint achieved, or last completed ratio; n=7–11 per age. *Different from adult rats, p<0.05 (Tukey's HSD post hoc test).
When the response requirement increased to FR5, adult rats maintained their self-administration of nicotine whereas adolescent intake significantly decreased (F(1,24)=18.00, p<0.001). There was an overall increase in the number of infusions earned across the FR5 sessions (F(5,116)=8.40, p<0.001); this effect was independent of age (p>0.05).
Figure 1b presents mean (±SEM) number of nicotine infusions earned during the dose–response determination. Overall, the adult rats continued to self-administer more nicotine than adolescents (F(1,24)=8.58, p<0.01). A significant main effect of Infusion Dose (F(2,46)=3.32, p<0.05) indicated that as the infusion dose increased, self-administration decreased. An Age × Infusion Dose interaction (F(2,46)=4.07, p<0.05) showed that only adult rats demonstrated dose-dependent decreases in nicotine intake with increasing infusion dose, whereas for the adolescents, the dose–response curve remained relatively flat. Adolescents earned significantly fewer nicotine infusions at the lower doses compared with adults (p<0.05), but not at the highest dose.
Figure 1c presents the mean (±SEM) number of nicotine infusions earned (0.03 mg/kg/infusion) and median breakpoints achieved during the three PR sessions. Adult rats earned significantly more nicotine infusions than the rats that initiated nicotine self-administration as adolescents (‘adolescent-onset’; Figure 3, F(1,13)=8.95, p<0.05), and this effect remained stable across sessions (p>0.05). The results of the breakpoint analysis supported that obtained from the analysis of infusions earned. The adult rats achieved significantly higher breakpoints compared with adolescent-onset rats; this was significant for the first and third sessions (p<0.05) and approached significance during the second session (p=0.058).
Extinction and reinstatement of nicotine seeking in rats that initiated nicotine self-administration during adolescence and adulthood. (a) Mean±SEM number of responses on the previously active lever during the last session at FR5 and the first 10 sessions of extinction. During this phase, lever responding resulted in saline infusions and the presentation of the compound light-tone stimulus previously paired with nicotine infusions. *Different from adolescent rats, p<0.05 (Tukey's HSD post hoc test) (b), Mean±SEM number of responses on the previously active lever during the tests for reinstatement of nicotine seeking induced by priming injections of nicotine (0.075, 0.15, 0.3 mg/kg, s.c.). Responding on the previously active lever during reinstatement was not reinforced with nicotine; n=16–18 per age. *Different from age-appropriate vehicle control, p<0.05 (Tukey's HSD post hoc test). Note the use of different scales on the y axis.
Experiment 2: Nicotine Self-Administration, Extinction, and Reinstatement
As in Experiment 1, adolescent and adult rats earned a similar number of nicotine infusions under the FR1 reinforcement schedule (Figure 2a, p>0.05), and performance was stable across the five sessions (p>0.05), and did not vary as a function of age (p>0.05). At the FR2 schedule, adolescents and adults performed similarly (p>0.05), with the exception of adults earning more nicotine infusions during the second session (p<0.01); otherwise, intake was similar across the four sessions at FR2 (p>0.05).
Nicotine self-administration in adolescent and adult rats during Experiment 2. (a) Mean±SEM number of nicotine infusions earned during the first nine sessions of nicotine self-administration under FR1 and FR2 schedules of reinforcement. (b) Mean±SEM number of nicotine infusions earned during four 2 h sessions under a progressive ratio schedule of reinforcement (bars) and median breakpoints, or last completed ratio achieved (lines); n=16–18 per age. *Different from adult rats, p<0.05 (Tukey's HSD post hoc test).
PR responding
As shown in Figure 2b, clear age differences in the mean (±SEM) number of nicotine infusions earned emerged when the PR schedule was introduced, with adults earning more nicotine infusions compared with adolescents (F(1,32)=17.46), p<0.001). The number of infusions earned increased across sessions (F(3,96)=11.73, p<0.001), but this increase was restricted to adult rats (F(3,96)=7.71, p<0.001). Analysis of breakpoints indicated that over all four sessions, the adult rats achieved higher breakpoints than the adolescent rats (p<0.05).
Extinction
Figure 3a presents the mean (±SEM) number of active lever responses during the last FR5 session and the first 10 extinction sessions. Because of the higher baseline responding in adult rats, active lever responding on the last FR5 session was used as a covariate in the analysis of extinction sessions. Rate of extinction, as measured by the decline in responses made on the nicotine-associated lever, varied as a function of age (Session × Age: F(9,243)=3.66, p<0.001); responding on the nicotine-associated lever remained higher in adults compared with adolescent-onset rats during the first two extinction sessions (p<0.05), but this age difference disappeared by the third extinction session.
Nicotine priming-induced reinstatement
The rats in both age groups attained extinction criterion by session 18 (median). Nicotine priming reinstated responding on the nicotine-associated lever in a dose-dependent manner (Figure 3b, F(3,69)=20.95, p<0.001). No age differences in nicotine priming-induced reinstatement were observed (p>0.05). Analysis within each age group revealed that only the two higher doses (0.15 and 0.3 mg/kg) reinstated nicotine seeking compared to age-appropriate vehicle controls.
Experiment 3: Saccharin Self-Administration, Dose–Response, Extinction, and Reinstatement
Mean (±SEM) number of saccharin reinforcements earned and amount consumed (ml/kg) at each FR schedule are presented in Table 1. The large discrepancy in reinforcements earned in earlier sessions may partially be explained by the large difference in body weight between adolescent (P33: 96.6±2.5 g) and adult (P94–99: 387.5±3.5 g) rats, as differences in body size could attribute to differences in absolute levels of consumption. In consideration of this, we also conducted statistical analyses of saccharin consumption based on body weight (ml/kg). Under FR1 and FR2 conditions, adolescents earned fewer saccharin reinforcements than adults (FR1: F(1,30)=31.93, p<0.001; FR2: F(1,26)=9.48, p<0.01), but their intake based on body weight was similar (p>0.05). Intake declined in both age groups across the FR1 sessions (F(5,146)=4.31, p<0.001), but remained stable during the FR2 sessions (p>0.05). When the reinforcement schedule was increased to FR5, adolescent and adult rats earned a similar number of saccharin reinforcements (p>0.05). The number of saccharin reinforcements earned increased across sessions in adolescent, but not adult rats (F(5,130)=3.40, p<0.01). Examination of saccharin intake based on body weight indicated that adolescents consumed more saccharin compared with adults (F(1,26)=30.66, p<0.001).
Concentration–response
During the concentration–response determination, adolescent and adult rats earned a similar number of saccharin reinforcements (p>0.05) although intake was greater in the former group (F(1,26)=13.85, p<0.001; Table 1). A significant main effect of saccharin concentration emerged (F(3,78)=34.21, p<0.001), indicating that as saccharin concentration increased, reinforcements earned increased, and this effect was independent of age (p>0.05). The number of reinforcements earned was similar at the two lower concentrations, that is, 0.025 and 0.05%, and increased significantly at the 0.1 and 0.2% concentrations.
PR responding
Figure 4a presents mean (±SEM) number of saccharin reinforcements earned and median breakpoints achieved during the three PR sessions. Rats that initiated saccharin self-administration as adolescents and adults earned a similar number of saccharin reinforcements (p>0.05) and both age groups demonstrated a significant decline in reinforcements earned over the three PR sessions (F(2,52)=22.23, p<0.001). Analysis of breakpoint also revealed no significant age difference (p>0.70).
Saccharin self-administration under progressive ratio conditions in adolescent and adult rats. Mean (±SEM) number of saccharin reinforcements earned during 2 h PR sessions (bars) and median breakpoints, or last completed ratio achieved (lines) in (a), rats that initiated saccharin self-administration as adolescents (adolescent-onset) and adults in Experiment 3, n=14 per age, (b) adolescent and adult rats in Experiment 4, n=8 per age.
Extinction
Figure 5 presents the mean (±SEM) number of active lever responses during the last FR5 session and the first eight extinction sessions. Responding on the saccharin-associated lever declined significantly across extinction sessions (F(9,227)=23.46, p<0.001), and this was independent of age (p>0.05).
Extinction of saccharin seeking in rats that initiated self-administration during adolescence and adulthood. Mean±SEM number of responses on the previously active lever during the last session at FR5 and the first eight sessions of extinction. During this phase, lever responding resulted in the presentation of the compound light-tone stimulus previously paired with saccharin delivery and was not reinforced with saccharin; n=14 per age.
Experiment 4: Saccharin Self-Administration Under a PR Schedule
Figure 4b presents mean (±SEM) number of saccharin reinforcements earned and median breakpoints achieved during the two PR sessions. Adolescent and adult rats earned a similar number of saccharin reinforcements and analysis of breakpoint also revealed no significant age difference (both, p>0.05).
DISCUSSION
The current study examined three measures of nicotine-taking behavior in rats that were trained to self-administer nicotine during adolescence or adulthood. First, we used a drug self-administration procedure under fixed-ratio and PR schedules that has been employed to assess the reinforcing effects of nicotine and other drugs (Richardson and Roberts, 1996; Picciotto and Corrigall, 2002; Wise, 2004). We then used a reinstatement procedure to assess relapse to drug seeking induced by acute re-exposure to the self-administered drug or other stimuli following extinction of nicotine-maintained responding (Stewart, 2000; Le and Shaham, 2002; Shaham et al, 2003; Weiss, 2005). Using these tests, we did not find evidence for increased vulnerability to nicotine-taking behavior in adolescent rats. On the contrary, on several measures (PR, FR5, and extinction responding), lever responding of the rats trained for nicotine self-administration during adolescence was significantly lower than the rats that were trained to self-administer the drug during adulthood. The present data from established rat models of drug self-administration and drug relapse suggest that age-dependent psychosocial differences, rather than biological differences in the rewarding effects of nicotine, likely account for the high rates of initiation of cigarette smoking in adolescents.
Nicotine Self-Administration in Adolescent and Adult Rats
We examined potential differences in the reinforcing effects of nicotine between adolescent and adult rats in the intravenous self-administration procedure. Under conditions of low response cost (FR1 or FR2 schedules), adolescent and adult rats self-administered nicotine at similar rates, indicating that nicotine acts as a positive reinforcer in both age groups. In contrast, at higher response costs (FR5 or PR schedules), nicotine self-administration was higher in adult than in adolescent rats. This age difference appears specific to nicotine, since it did not occur with a non-drug reinforcer, saccharin.
These present findings with the FR1 and FR2 reinforcement schedules are consistent with the study by Belluzzi et al (2005) in which no age differences for nicotine self-administration were observed under the FR1 reinforcement schedule. Our results are also partly consistent with the study by Levin et al (2003), in which adolescent and adult female rats earned a similar number of nicotine infusions during the early phase of training, when the younger rats were in late adolescence (P43–46).
Adult rats showed a dose-dependent decrease in nicotine self-administration, a finding consistent with previous reports (Corrigall and Coen, 1989; Shoaib et al, 1997; Watkins et al, 1999; Le et al, 2006). In contrast, increasing the nicotine dose had minimal effect on responding in adolescent rats. The flattened dose–response curve for the adolescents suggests that, compared with adults, they may be insensitive to changing doses of nicotine, or that the nicotine dose–response curve is shifted in these younger animals. The upward shift in the dose–response curve for the adult rats may reflect increased reinforcing effects of nicotine in the adult rats (Piazza et al, 2000). Alternatively, the increase in infusions earned by adults at the lower nicotine doses may result from reduced nicotine reinforcement, causing a rightward shift in the dose–response curve. This is unlikely, however, since the results from the PR testing are inconsistent with the idea that nicotine is less reinforcing in adults than in adolescents.
One explanation of the findings with the FR5 and PR reinforcement schedules is that adolescents might be less capable of performing under higher response costs due to the greater physical effort required. To address this, experiments using saccharin as the reinforcer were conducted. Under low-response requirements, adult rats earned more saccharin reinforcements compared with adolescents. This is likely attributable to the smaller body size, and thus, consummatory limitations of the adolescent rats. Saccharin intake (ml/kg), however, was similar across age groups. Upon increasing the schedule to FR5, both adolescents and adults lever pressed similarly for saccharin and earned a similar number of saccharin reinforcements in Experiments 3 and 4. These results suggest that our findings with nicotine are not due to age-dependent differences in performance. This possibility, however, cannot be ruled out completely since both age groups exhibited decreases in the number of saccharin reinforcers earned when the reinforcement schedule was increased and PR responding for saccharin was lower than that for nicotine.
The higher nicotine intake in adult rats may be attributable to a greater nicotine-induced enhancement of the rewarding effects of the compound (light+tone) cue associated with nicotine delivery (Caggiula et al, 2001). While not specifically tested, the similarity in nicotine priming-induced reinstatement across age argues against this possibility (see below).
The finding that nicotine may be less reinforcing in adolescent than in adult rats, as measured in the self-administration procedure is surprising in light of previous studies using CPP and CTA procedures. The results of these studies suggest that nicotine is more rewarding and less aversive in adolescents compared with adults (Vastola et al, 2002; Belluzzi et al, 2004; Torrella et al, 2004; Wilmouth and Spear, 2004; Shram et al, 2006). There are several possible reasons for this potential discrepancy. In the CPP and CTA studies, nicotine is injected noncontingently via the subcutaneous route, while in the self-administration studies, nicotine is earned contingently via the intravenous route. The intravenous injections would result in a more rapid increase in brain levels of nicotine than the subcutaneous route and it has previously been shown that the more rapidly nicotine or other drugs reach the brain, the greater their abuse liability (Shoaib, 1996). There is also evidence in studies using adult rats that contingent and noncontingent drug exposures have different effects on brain and behavior (Wilson et al, 1994; Dworkin et al, 1995; Jacobs et al, 2003). In addition, it is not surprising that different results are obtained in a classical conditioning CPP procedure and an operant self-administration procedure; there is evidence in the literature for both similarities and differences in the anatomical substrates of the reinforcing effects of drugs, as measured in the two procedures (Bardo and Bevins, 2000).
Extinction and Reinstatement of Nicotine Seeking in Adolescent and Adult-Onset Rats
Early onset of tobacco use is associated with a reduced probability of quitting and higher rates of relapse (Breslau and Peterson, 1996; Chen and Millar, 1998; Cui et al, 2006). In the current study, we assessed relapse to nicotine seeking, as measured in extinction and reinstatement tests, in rats that initiated nicotine self-administration as adolescents (adolescent-onset) or adults (adult-onset).
Adult-onset rats demonstrated greater resistance to extinction under saline substitution conditions when compared with adolescent-onset rats. This observation is consistent with our finding of a greater reinforcing efficacy of nicotine in adult rats under the PR schedule and extends the findings by Donny et al (2004) and Roth and Carroll (2004) relating rate of acquisition of drug self-administration and breakpoint. The greater resistance to extinction also potentially suggests that the compound cue associated with nicotine delivery acquired greater conditioned reinforcing effects in the adult rats than in the adolescent rats. However, the greater number of nicotine-cue pairings in the adult rats may also have contributed to this effect.
Unlike extinction responding, no age differences were observed in the effect of nicotine priming on reinstatement of drug seeking. For both age groups, nicotine priming injections reliably reinstated nicotine seeking, an observation consistent with previous reports (Shaham et al, 1997; Le et al, 2006). The mechanisms underlying nicotine-induced reinstatement are unknown. Stewart et al (1984) suggested that drug priming restores the incentive value of extinguished drug-associated cues, resulting in resumption of drug seeking. Alternatively, nicotine priming may enhance responding for the compound cue, which can have intrinsic reinforcing properties of its own (Caggiula et al, 2001; Olausson et al, 2004; Chaudhri et al, 2005), rather than increasing nicotine seeking per se. The former interpretation may be more likely because the effect of nicotine priming injections on reinstatement of lever responding is to some degree stimulus specific. While there is evidence that nicotine can reinstate alcohol seeking (Le et al, 2003) and cocaine seeking in alcohol-preferring P rats (Le et al, 2006), it does not reinstate cocaine seeking in alcohol nonpreferring (NP) rats (Le et al, 2006) or other rat strains (Wise et al, 1990; Schenk and Partridge, 1999). Also, nicotine-priming injections do not reinstate food seeking (Shaham et al, 1997) and, surprisingly, attenuate the reinstatement of methamphetamine seeking (Hiranita et al, 2004).
Our data suggest that age of onset of nicotine self-administration does not influence nicotine priming-induced reinstatement of nicotine seeking during adulthood. It remains to be seen if cue or stress-induced reinstatement of nicotine seeking is differentially affected by age at onset of nicotine self-administration. In addition, it would be interesting to test extinction and reinstatement during the adolescent period; the ability to investigate this possibility is limited by the brevity of the adolescent period.
Concluding Remarks
In the present series of experiments, we did not find evidence for an enhanced vulnerability to the rewarding and relapse-provoking effects of nicotine in adolescent rats. These findings are in apparent contrast to the epidemiological literature on increased vulnerability to nicotine addiction in humans during adolescence. These findings suggest that age-dependent psychosocial and behavioral differences (Spear, 2000; Simons-Morton et al, 2001; Deakin et al, 2004; Kelley et al, 2004), rather than biological differences in the rewarding effects of nicotine, likely account for the high rates of initiation of cigarette smoking in adolescents. This is a likely conclusion because in generalizing the present findings to humans, it is important to note that the subjects in our experiments were randomly assigned to age of onset of nicotine self-administration. In contrast, in human studies, vulnerable individuals are likely to initiate smoking during adolescence and, therefore, are unlikely to be represented in a sample of adult-onset smokers.
Albeit surprising, our findings that adolescent rats are less willing to work for nicotine at high response costs agree with the results of a number of studies in humans. Interestingly, the adolescent age group has been shown to be the most sensitive to increases in cigarette prices (Ding, 2003) and they are also the most likely age group to seek noncommercial (and easier to obtain) sources of cigarettes (Castrucci et al, 2002). Although our findings do not indicate an enhanced vulnerability to the reinforcing effects of nicotine during adolescence, repeated exposure to nicotine during this vulnerable stage has been shown to produce long-term neurobehavioral consequences (Trauth et al, 1999, 2001; Adriani et al, 2003).
References
Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB et al (2003). Evidence for enhanced vulnerability to nicotine during periadolescence in rats. J Neurosci 23: 4712–4716.
Arnett J (1992). Reckless behavior in adolescence: a developmental perspective. Dev Rev 12: 339–373.
Bardo MT, Bevins RA (2000). Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 153: 31–43.
Belluzzi JD, Lee AG, Oliff HS, Leslie FM (2004). Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology (Berl) 174: 389–395.
Belluzzi JD, Wang R, Leslie FM (2005). Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology 30: 705–712.
Breslau N, Peterson EL (1996). Smoking cessation in young adults: age at initiation of cigarette smoking and other suspected influences. Am J Public Health 86: 214–220.
Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA et al (2001). Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav 70: 515–530.
Castrucci BC, Gerlach KK, Kaufman NJ, Orleans CT (2002). Adolescents' acquisition of cigarettes through noncommercial sources. J Adolesc Health 31: 322–326.
Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA et al (2005). Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology (Berl) 180: 258–266.
Chen H, Matta SG, Sharp BM (2007). Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology 32: 700–709.
Chen J, Millar WJ (1998). Age of smoking initiation: implications for quitting. Health Rep 9: 39–46 (English); 39–48 (French).
Clark DB, Kirisci L, Moss HB (1998). Early adolescent gateway drug use in sons of fathers with substance use disorders. Addict Behav 23: 561–566.
Coogan PF, Adams M, Geller AC, Brooks D, Miller DR, Lew RA et al (1998). Factors associated with smoking among children and adolescents in Connecticut. Am J Prev Med 15: 17–24.
Corrigall WA, Coen KM (1989). Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 99: 473–478.
Cui Y, Wen W, Moriarty CJ, Levine RS (2006). Risk factors and their effects on the dynamic process of smoking relapse among veteran smokers. Behav Res Ther 44: 967–981. E-pub 8 September 2005.
Deakin J, Aitken M, Robbins T, Sahakian BJ (2004). Risk taking during decision-making in normal volunteers changes with age. J Int Neuropsychol Soc 10: 590–598.
Depoortere RY, Li DH, Lane JD, Emmett-Oglesby MW (1993). Parameters of self-administration of cocaine in rats under a progressive-ratio schedule. Pharmacol Biochem Behav 45: 539–548.
DeWit DJ, Offord DR, Wong M (1997). Patterns of onset and cessation of drug use over the early part of the life course. Health Educ Behav 24: 746–758.
Ding A (2003). Youth are more sensitive to price changes in cigarettes than adults. Yale J Biol Med 76: 115–124.
Donny EC, Caggiula AR, Mielke MM, Booth S, Gharib MA, Hoffman A et al (1999). Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 147: 135–142.
Donny EC, Lanza ST, Balster RL, Collins LM, Caggiula A, Rowell PP (2004). Using growth models to relate acquisition of nicotine self-administration to break point and nicotinic receptor binding. Drug Alcohol Depend 75: 23–35.
Dworkin SI, Mirkis S, Smith JE (1995). Response-dependent versus response-independent presentation of cocaine: differences in the lethal effects of the drug. Psychopharmacology (Berl) 117: 262–266.
Eissenberg T, Balster RL (2000). Initial tobacco use episodes in children and adolescents: current knowledge, future directions. Drug Alcohol Depend 59 (Suppl 1): S41–S60.
Epstein DH, Preston KL, Stewart J, Shaham Y (2006). Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 189: 1–16. E-pub 22 September 2006.
Frantz KJ, O'Dell LE, Parsons LH (2007). Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology 32: 625–637.
Harvey DM, Yasar S, Heishman SJ, Panlilio LV, Henningfield JE, Goldberg SR (2004). Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology (Berl) 175: 134–142.
Hiranita T, Anggadiredja K, Fujisaki C, Watanabe S, Yamamoto T (2004). Nicotine attenuates relapse to methamphetamine-seeking behavior (craving) in rats. Ann NY Acad Sci 1025: 504–507.
Jacobs EH, Smit AB, de Vries TJ, Schoffelmeer AN (2003). Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci 24: 566–573.
Kandel DB, Yamaguchi K, Chen K (1992). Stages of progression in drug involvement from adolescence to adulthood: further evidence for the gateway theory. J Stud Alcohol 53: 447–457.
Kelley AE, Schochet T, Landry CF (2004). Risk taking and novelty seeking in adolescence: introduction to part I. Ann NY Acad Sci 1021: 27–32.
Le AD, Li Z, Funk D, Shram M, Li TK, Shaham Y (2006). Increased vulnerability to nicotine self-administration and relapse in alcohol-naive offspring of rats selectively bred for high alcohol intake. J Neurosci 26: 1872–1879.
Le AD, Wang A, Harding S, Juzytsch W, Shaham Y (2003). Nicotine increases alcohol self-administration and reinstates alcohol seeking in rats. Psychopharmacology (Berl) 168: 216–221. E-pub 21 January 2003.
Le A, Shaham Y (2002). Neurobiology of relapse to alcohol in rats. Pharmacol Ther 94: 137–156.
Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS (2003). Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology (Berl) 169: 141–149.
Olausson P, Jentsch JD, Taylor JR (2004). Nicotine enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 171: 173–178.
Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M (2000). Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci 20: 4226–4232.
Picciotto MR, Corrigall WA (2002). Neuronal systems underlying behaviors related to nicotine addiction: neural circuits and molecular genetics. J Neurosci 22: 3338–3341.
Richardson NR, Roberts DC (1996). Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66: 1–11.
Risner ME, Goldberg SR (1983). A comparison of nicotine and cocaine self-administration in the dog: fixed-ratio and progressive-ratio schedules of intravenous drug infusion. J Pharmacol Exp Ther 224: 319–326.
Roth ME, Carroll ME (2004). Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology (Berl) 172: 443–449. E-pub 4 December 2003.
Schenk S, Partridge B (1999). Cocaine-seeking produced by experimenter-administered drug injections: dose-effect relationships in rats. Psychopharmacology (Berl) 147: 285–290.
Shaham Y, Adamson LK, Grocki S, Corrigall WA (1997). Reinstatement and spontaneous recovery of nicotine seeking in rats. Psychopharmacology (Berl) 130: 396–403.
Shaham Y, Shalev U, Lu L, De Wit H, Stewart J (2003). The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 168: 3–20. E-pub 26 October 2002.
Shoaib M (1996). Determinants of nicotine self-administration. Drug Dev Res 38: 212–221.
Shoaib M, Schindler CW, Goldberg SR (1997). Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology (Berl) 129: 35–43.
Shoaib M, Stolerman IP (1999). Plasma nicotine and cotinine levels following intravenous nicotine self-administration in rats. Psychopharmacology (Berl) 143: 318–321.
Shram MJ, Funk D, Li Z, Le AD (2006). Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berl) 186: 201–208.
Simons-Morton B, Haynie DL, Crump AD, Eitel SP, Saylor KE (2001). Peer and parent influences on smoking and drinking among early adolescents. Health Educ Behav 28: 95–107.
Spear LP (2000). The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24: 417–463.
Spear LP, Brake SC (1983). Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol 16: 83–109.
Stafford D, LeSage MG, Glowa JR (1998). Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology (Berl) 139: 169–184.
Stewart J (2000). Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiatry Neurosci 25: 125–136.
Stewart J, de Wit H, Eikelboom R (1984). Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev 91: 251–268.
Torrella TA, Badanich KA, Philpot RM, Kirstein CL, Wecker L (2004). Developmental differences in nicotine place conditioning. Ann NY Acad Sci 1021: 399–403.
Trauth JA, Seidler FJ, Ali SF, Slotkin TA (2001). Adolescent nicotine exposure produces immediate and long-term changes in CNS noradrenergic and dopaminergic function. Brain Res 892: 269–280.
Trauth JA, Seidler FJ, McCook EC, Slotkin TA (1999). Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res 851: 9–19.
Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP (2002). Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav 77: 107–114.
Watkins SS, Epping-Jordan MP, Koob GF, Markou A (1999). Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav 62: 743–751.
Weiss F (2005). Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol 5: 9–19.
Wilmouth CE, Spear LP (2004). Adolescent and adult rats' aversion to flavors previously paired with nicotine. Ann NY Acad Sci 1021: 462–464.
Wilson JM, Nobrega JN, Corrigall WA, Coen KM, Shannak K, Kish SJ (1994). Amygdala dopamine levels are markedly elevated after self- but not passive-administration of cocaine. Brain Res 668: 39–45.
Wise RA (2004). Dopamine, learning and motivation. Nat Rev Neurosci 5: 483–494.
Wise RA, Murray A, Bozarth MA (1990). Bromocriptine self-administration and bromocriptine-reinstatement of cocaine-trained and heroin-trained lever pressing in rats. Psychopharmacology 100: 355–360.
Yu J, Williford WR (1992). The age of alcohol onset and alcohol, cigarette, and marijuana use patterns: an analysis of drug use progression of young adults in New York State. Int J Addict 27: 1313–1323.
Acknowledgements
This work was supported by a grant from the National Institute on Alcohol Abuse and Alcoholism to ADL. MJS was supported by a Natural Sciences and Engineering Research Council postgraduate scholarship and a Canadian Institute for Health Research Strategic Training Programme for Tobacco Use in Special Populations scholarship. We thank Dr Yavin Shaham for his helpful comments during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure/Conflict of Interest
We declare that no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional services and there are no personal financial holdings that may be perceived as constituting a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Shram, M., Funk, D., Li, Z. et al. Nicotine Self-Administration, Extinction Responding and Reinstatement in Adolescent and Adult Male Rats: Evidence Against a Biological Vulnerability to Nicotine Addiction during Adolescence. Neuropsychopharmacol 33, 739–748 (2008). https://doi.org/10.1038/sj.npp.1301454
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301454
Keywords
This article is cited by
-
Individual variations in motives for nicotine self-administration in male rats: evidence in support for a precision psychopharmacology
Translational Psychiatry (2024)
-
Factors influencing JUUL e-cigarette nicotine vapour-induced reward, withdrawal, pharmacokinetics and brain connectivity in rats: sex matters
Neuropsychopharmacology (2023)
-
Adolescent nicotine and footshock exposure augments adult nicotine self-administration and drug-seeking without affecting baseline anxiety-like behaviour or stress responsivity in male rats
Psychopharmacology (2021)
-
Role of trace amine-associated receptor 1 in nicotine’s behavioral and neurochemical effects
Neuropsychopharmacology (2018)
-
Effects of alcohol dependence on discrete choice between alcohol and saccharin
Neuropsychopharmacology (2018)