Abstract
Drugs of abuse, including, nicotine have been shown to enhance brain reward functions in the mesocortico-limbic dopamine (DA) system in general, and the nucleus accumbens in particular. The latter occupies a prominent position in the ventral striatum and expresses a high density of DA D3 receptors. As such, the present study aimed at investigating the effect of the selective D3 receptor antagonist SB-277011-A on both the stable maintenance of intravenous nicotine self-administration and nicotine-triggered relapse to nicotine-seeking behavior in the rat. SB-277011-A (3–10 mg/kg i.p.) significantly reduced reinstatement of nicotine-seeking behavior without affecting nicotine self-administration per se. These results suggest that DA D3 receptors are involved in the reinstatement of nicotine-seeking behavior independently of any interaction with the primary reinforcing effects of nicotine itself. These findings point toward the potential use of selective DA D3 receptor antagonists for the pharmacotherapeutic management of relapse to drug-seeking behaviors.
Similar content being viewed by others
INTRODUCTION
Nicotine and the vast majority of abused drugs, including cocaine, amphetamine, morphine, ethanol, phencyclidine, and Δ9-tetrahydrocannabinol have the essential commonality of enhancing basal neuronal firing and/or basal neurotransmitter release in the mesolimbic dopamine (DA) system (Di Chiara and Imperato, 1988; Koob and Le Moal, 1998). Specifically, the neurobiological mechanisms underlying dependence on drugs of abuse appear to be associated with a sustained increase in the release of DA into the extracellular space of the nucleus accumbens (NAc) (Berridge and Robinson, 1998; Di Chiara, 1995). Thus, activation of DA neurotransmission in the NAc seems to be a key component underlying the reward and/or incentive motivation produced by drugs of abuse (Berridge and Robinson, 1998; Wise and Rompre, 1989).
Although the role of DA D1 and D2 receptors in mediating the addictive liability of drugs has been fairly well characterized (see for example Caine et al, 2002; Haile and Kosten, 2001; Hummel and Unterwald, 2002), a growing body of evidence has recently strengthened the likelihood that the DA D3 receptor is significantly involved in mechanisms of drug dependence and abuse. For example, the density of DA D3 receptors is elevated one- to three-fold in the NAc and ventromedial subregions of the caudate-putamen in the brains of cocaine-overdose fatalities compared with both age-matched, drug-free control subjects and cocaine-overdose victims presenting preterminal excited delirium (Mash and Staley, 1999; Staley and Mash, 1996). Furthermore, the expression of DA D3 receptor mRNA in the human NAc is increased six-fold in cocaine-overdose victims (Segal et al, 1997). Recent studies also showed a selective increase in the DA D3 receptor binding and D3 receptor mRNA in the shell of the NAc of nicotine-sensitized rats without any significant changes in the expression of DA D1 and D2 receptors (Le Foll et al, 2003). These findings are in line with the distribution of DA receptors, which does not follow a homogeneous pattern throughout the NAc. Contrary to DA D1 and D2 receptors, DA D3 receptors are expressed preferentially in medium-sized spiny neurons of the rostral and ventromedial shell of the NAc and in granule cells of the island of Calleja major (ICjM), regions in which the D2 receptors are scarcely expressed (Bouthenet et al, 1991; Diaz et al, 1995; Sokoloff et al, 1990). The distribution of the D3 receptor in the human brain also appears to follow a rather similar pattern to that observed in the rat brain (Gurevich and Joyce, 1999; Hall et al, 1996; Landwehrmeyer et al, 1993; Murray et al, 1994; Shafer and Levant, 1998; Suzuki et al, 1998).
The precise role of the D3 receptor in drug dependence processes has, however, been significantly hampered by the lack of pharmacological tools showing significant selectivity for DA D3 over D2 receptors. For example, most compounds developed so far have a 10- to 30-fold selectivity for DA D3 over D2 receptors in vivo(Audinot et al, 1998; Sautel et al, 1995; Waters et al, 1994). In addition, the partial agonist BP 897 is also a potent and selective antagonist at both DA D3 and D2 receptors (Wood et al, 2000). Altogether these findings emphasize the need for selective tools to investigate the role of the DA D3 receptor in drug dependence. In contrast with most DA D3 antagonists developed so far, the recent DA D3 receptor antagonist SB-277011-A (trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2-yl)ethyl]cyclohexyl]-4-quinolininecarboxamide) shows high affinity and 100-fold selectivity for D3 over D2 receptors and 66 other receptors, enzymes, and ion channels (Reavill et al, 2000; Stemp et al, 2000). The selective DA D3 antagonist SB-277011-A has been shown to be efficacious against cocaine-seeking behavior and cocaine-induced conditioned hyperactivity, but does not seem to play a role in responding directly reinforced by cocaine (Di Ciano et al, 2003; Le Foll et al, 2002). Furthermore, SB-277011-A blocks cocaine-induced enhancement of electrical brain stimulation reward, attenuates both the development and expression of cocaine-induced place preference, and decreases cocaine-triggered relapse to cocaine-seeking behavior in a dose-dependent manner (Vorel et al, 2002). Accordingly, the present experiments were undertaken to assess the effect of acute systemic antagonism at DA D3 receptors by using SB-277011-A on both the stable maintenance of intravenous nicotine self-administration and nicotine-triggered relapse to nicotine-seeking behavior.
MATERIALS AND METHODS
Animals
Male Wistar rats (Charles River, Germany) were individually housed in a temperature-controlled environment with lights on from 06.00 to 18.00 h. During the experiments, water was continuously available and animals were maintained at a constant body weight of 240–260 g (85% of their ad libitum body weight). All experiments were pre-reviewed and consented by a local animal care committee in complete accordance with the guidelines of the ‘Principles of laboratory animal care’ (NIH publication No. 86–23, revised 1985) as well as with the regulations of the Italian laws.
Apparatus
Behavioral testing (self-administration sessions and reinstatement phase) was conducted in 16 operant chambers(Coulbourn Instruments, Leigh Valley, USA) encased in sound-insulated cubicles, equipped with a ventilation fan (Ugo Basile, Comerio, Italy) that also screened external noise. Each chamber was equipped with two levers on the front panel, located 12.5 cm apart and 2 cm above the grid floor. A food magazine was situated in an opening in the panel between the two levers, 1 cm above the floor. A 2-W white light was located 26 cm above the food magazine and was activated throughout the entire session. Presses on the right lever (RLP or ‘active lever pressing’), corresponding to the fixed-ratio values required by the schedules of reinforcement, produced the delivery of 45 mg food pellets (Bioserv, Frenchtown, NJ, USA) or the activation of the infusion pump, except during extinction and reinstatement conditions. Delivery of reinforcement was signaled by the 1-s illumination of a 4-W white stimulus light located in the same hole of the food magazine and by 1-s sounding of a Sonalert device (2.9 kHz, 80 dB). Nicotine solution was administered via an infusion pump (Model A-99Z, Razel Scientific Instruments Inc., CT, USA) at a volume of 0.022 ml over a 1-s period. Presses on the left lever (LLP) did not have any consequence. Both lever presses and reinforcement deliveries were recorded. Data acquisition and schedule parameters were controlled by Med-PC software (Med Associates Inc., Georgia, GA) running on two Compaq microcomputers interfaced with the chambers via interface modules (Med Associates Inc.).
Experimental Procedures
Training to lever-press for food reinforcement
Following a 24-h deprivation period, rats were trained to press the right lever for food as a reinforcer. The procedure was initiated under a fixed-ratio (FR-1) schedule of reinforcement with a time-out period of 60-s (TO 60s). Each FR-1 session lasted until rats had received 100 pellets or was terminated following a maximal 2-h period. Rats were then gradually trained up to a final FR-2 schedule with a TO 60s over a 2-h period. Each FR-2 session was terminated after 2 h. Rats underwent surgery once they reached the criterion by which the ratio between right lever presses and number of reinforcement was less than or equal to 4. This number represents a measure of the selectivity of rats' responding, so that the smaller the ratio, the higher the level of responding specifically directed toward obtaining food reinforcement.
Surgery
Rats were anesthetized with pentobarbital sodium (50 mg/kg; Siegfried Zofingen Switzerland) 1 ml/kg i.p. (5-min pretreatment with atropine sulfate 0.5 mg/kg I.P.; Sigma, St Louis, MO, USA). They were then implanted with a silastic catheter in the right jugular vein. The free end of the catheter was connected to a modified C313G cannula assembly (Plastic One, VA). The resulting unit was mounted onto the skull with dental acrylic cement and fixed via tissue adhesive (3M Animal Care Products, St Paul, MN, USA).
Animals received one bolus injection of 0.250 ml of an antibiotic suspension of terramicine (Terramicina long-acting, Pfizer Inc., NY, USA) for a 72-h protection. Animals were then injected i.v. with 0.1 ml of a solution containing 4 UI/ml heparin (Liquemin, Roche S.p.A., Milano, Italy). This treatment was repeated twice a day for 5 days after surgery (recovery period).
Nicotine self-administration training
After the recovery period, rats were trained to nicotine self-administration on an FR-1 schedule of reinforcement. During this schedule each active lever press produced an infusion of nicotine (0.03 mg/kg/infusion) together with the activation of two conditioned stimuli, a light in the food magazine and a tone. Each infusion was followed by a 60s TO period during which responses were recorded, but had no scheduled consequences. Each session lasted until rats received 25 infusions of nicotine, or was terminated after a maximum of 3 h. If the animal met the criterion of 25 infusions within the end of the daily session, the FR value was increased to FR-2 the following day. For the FR-2 schedule of reinforcement, each session was terminated after a maximum of 2 h. This procedure lasted until rats reached at least 12 infusions of nicotine over the 2-h session for at least 3 consecutive days. The animals were then shifted to an FR-2 schedule over 1-h sessions. Responding was considered stable when the average inter-reinforcement time (IRT) did not vary more than 20% between three consecutive sessions. Stability criteria for responding to drugs of abuse such as cocaine are typically referred to as variability within parameters (eg IRT, number of active lever presses) comprised between 10 and 20% (Deroche-Gamonet et al, 2002; Goeders and Clampitt, 2002; Highfield et al, 2002). In contrast, criteria of responding stability for nicotine are generally fixed between 15 and 30% variability for at least three consecutive sessions (Corrigall et al, 2002; Paterson and Markou, 2002; Shoaib and Stolerman, 1999) because rats self-administering nicotine show higher variability in the pattern of responding compared with those obtaining cocaine. In our experimental conditions, the rate of responding was stable after about 3 weeks of training.
Reinstatement phase
Nicotine-seeking behavior was investigated 24 h after the last nicotine self-administration session. Rats showing stable nicotine self-administration were exposed to a multiple ‘relapse schedule’ consisting of two components. The first component was a 30-min phase during which animals were allowed to lever press under an FR-1 schedule. This instrumental response did not produce an infusion of nicotine as during training, but only the conditioned stimuli previously paired to nicotine self-administration. This stage of the test session was termed the ‘CS component’. At the end of the CS component, a noncontingent s.c. injection of nicotine (0.15 mg/kg) was delivered to rats and nicotine-paired lever presses were measured during the following 90-min phase. Lever pressing had no consequences. This second stage of the test session was termed the ‘priming component’ (Chiamulera et al, 1998). This model was designed to mimic the human situation of short-term, overnight withdrawal, when nicotine-seeking might be at its highest since the abstinence period allows for resensitization of nicotinic receptors, which in turn may result in increased positive reinforcement combined with withdrawal symptoms because of cholinergic hyperactivity. Experimental data confirm that the first cigarette of the day has enhanced reinforcing effects (Fagerstrom and Schneider, 1989). The exposure to nicotine-associated stimuli is also relevant to the human situation and gives a measure of short time extinction in the self-administration environment with the contingent presentation of conditioned stimuli. Unpublished experiments have shown that over the 30 min of the CS component there is a decrease in lever pressing compared with the preceding self-administration session, but this decrease is usually more profound if the CS presentations are removed. Moreover, this phase of the test gives a measure of nicotine seeking that is not dependent on the pharmacological and rate-altering effects of nicotine itself. After the nicotine priming, responding on the active lever did not lead to any infusion or CS presentations and represents a measure of nicotine seeking in the presence of a passive exposure to nicotine, which is also close to the human situation. The within-session extinction–reinstatement procedure has been previously used in other laboratories (see for example Schenk and Partridge, 1999; Self et al, 1996,1998), with two critical differences: (i) The extinction phase was immediately preceded by a self-administration session, thus adding confounding elements (see above) to the measure of behavior. (ii) The role of cues in maintaining responding was never measured, despite growing evidence that conditioned reinforcement may play a more important role in maintaining and inducing relapse to nicotine seeking for most drugs of abuse (Balfour et al, 2000; Caggiula et al, 2001).
Experimental Design
In a first series of experiments, the effect of SB-277011-A (0, 3, 10 mg/kg i.p.) was investigated against stable maintenance of nicotine self-administration per se. Once the rate of responding was stable according to the criteria described above, the animals were randomly treated with SB-277011-A according to a Latin Square Design. The following parameters were measured for each animal in each experimental group (n=8/group): the number of infusions/h, the active and inactive lever presses/h, and the IRT.
In a separate series of experiments, the effect of SB-277011-A (0, 3, 10 mg/kg i.p.) was assessed against nicotine-triggered relapse to nicotine-seeking behavior 24 h after the last nicotine self-administration session (see methodology above). The following parameters were measured for each animal in each experimental group (n=8/group): the number of active and inactive lever presses/h and the latency (s) to responding during both the cue and priming components of the drug-seeking test.
Drugs
Nicotine bitartrate (Sigma, St Louis, MO) used during self-administration sessions was dissolved in heparinized saline and the pH was adjusted to 7.4 with NaOH. Nicotine unit doses were expressed as mg of free base/kg of body weight/infusion. Nicotine bitartrate (Sigma, St Louis, MO) used as priming during the reinstatement phase was dissolved in physiological saline and the pH adjusted to 7.4 with NaOH. The nicotine dose of 0.15 mg/kg was expressed as mg of free base and given to rats subcutaneously in a volume of 1 ml/kg.
SB-277011-A (GlaxoSmithKline Pharmaceuticals, Harlow, UK) was dissolved in 10% hydroxypropyl-β-cyclodextrine (Sigma, St Louis, MO, USA) and administered in a volume of 1 ml/kg i.p. All solutions except nicotine bitartrate used for self-administration sessions were prepared immediately prior to use. Doses of SB-277011-A were chosen based on pharmacokinetic (Austin et al, 2001; Reavill et al, 2000) and electrophysiological (Ashby et al, 2000) characteristics as well as behavioural properties reported in previous studies (Di Ciano et al, 2003; Le Foll et al, 2002; Reavill et al, 2000; Vorel et al, 2002).
Statistical Analyses
Self-administration data were analyzed by using one-way analyses of variance (ANOVA) with repeated measurements. The differences between individual means were assessed with the post hoc Dunnett's test. Statistical significance was set at a probability level of P<0.05 for all tests.
RESULTS
Effect of SB-277011-A (3, 10 mg/kg i.p.) on Stable Maintenance of Nicotine Self-Administration
Rats trained for i.v. nicotine infusion acquired a robust self-administration behavior. SB-277011-A (0, 3, or 10 mg/kg i.p.) (n=8 rats/group) was administered 30 min prior to the beginning of the nicotine self-administration session. Each rat received all treatments spaced at least 2 days apart. SB-277011-A did not affect the number of infusions/60 min or the number of RLP/60 min compared with the vehicle group (F[2;14]=0.9, P=0.4 and F[2;14]=0.4, P=0.7, respectively) at any dose tested. Furthermore, SB-277011-A failed to modify significantly both the number of LLP/60 min as well as the IRT (F[2;14]=0.1, P=0.9 and F[2;14]=0.7, P=0.5).
Effect of SB-277011-A (3, 10 mg/kg i.p.) on Nicotine-Triggered Relapse to Nicotine Self-Administration
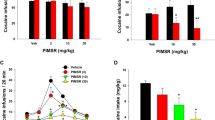
SB-277011-A (0, 3, 10 mg/kg i.p.) (n=8 rats/group) was administered 30 min before starting the relapse session. Responding was measured throughout the session up to 120 min (Figure 1). SB-277011-A (3, 10 mg/kg i.p.) did not significantly change responding during the first 30-min cue component (F[3;21]=0.3, P=0.8). Furthermore, SB-277011-A failed to affect inactive lever presses during the cue component of the relapse session (F[3;21]=0.6, P=0.6). In contrast, the acute systemic administration of nicotine (0.15 mg/kg s.c.) produced a significant increase in nicotine-paired lever presses in the vehicle/nicotine group compared with the vehicle/saline control group. Furthermore, SB-277011-A produced a significant reduction in nicotine-paired lever presses compared with the vehicle/nicotine group. A one-way ANOVA with repeated measurements over time revealed a significant difference in nicotine-paired lever presses (F[3;21]=5.2, P<0.01). The post hoc Dunnett's test confirmed significant effects of both doses of SB-277011-A (P<0.05) compared with responding in the vehicle/nicotine group (Figure 1). Finally, no significant changes in the number of inactive lever presses were seen during the priming component (F[3;21]=1.5, P=0.2).
Effect of SB-277011-A (0, 3, 10 mg/kg i.p.) on nicotine-triggered relapse to nicotine self-administration. Cumulative nicotine-paired lever presses were measured during the ‘cue’ and ‘nicotine’ components of responding, respectively. SB-277011-A was given 30 min prior to starting the relapse session. Nicotine (0.15 mg/kg s.c.) or saline (1 ml/kg s.c.) priming was given at the end of the ‘cue component’. Treatment groups as follows: veh/SAL (vehicle/saline), veh/NIC (vehicle/nicotine), 3 mg/NIC (SB-277011-A 3 mg/kg/nicotine), 10/NIC (SB-277011-A 10 mg/kg/nicotine). P<0.05; P<0.01. Dunnett's test vs veh/NIC.
Latency to receive a conditioned stimulus presentation and a first self-administered nicotine infusion provides a measurement of the motivation for these reinforcers. As such, the latency to responding during both cue and priming components was evaluated. The overall ANOVA failed to yield significant main effects. The average latency (s) to responding during the cue component for the vehicle/saline, vehicle/nicotine, and SB-277011-A (3, 10 mg/kg)/nicotine groups was 110.25±54.92, 81±23.06, 292.13±215.71, and 218.13±163.50 (mean±SEM, n=8 rats/group), respectively (F[3;21]=0.5, P=0.7). The average latency (s) to responding during the priming component for the vehicle/saline, vehicle/nicotine, and SB-277011-A (3, 10 mg/kg)/nicotine groups was 430.0±212.26, 163.63± 55.09, 170.13±58.94, and 406.63±198.27 (mean±SEM, n=8 rats/group), respectively (F[3;21]=1.01, P=0.4).
Finally, analysis of individual response patterns from representative rats revealed that increasing doses of SB-277011-A produced a decrease in response rates during the nicotine priming session (Figure 2).
Cumulative response records from a representative rat during cue- and nicotine-triggered relapse to nicotine-seeking behavior. SB-277011-A (3 and 10 mg/kg i.p.) or vehicle (veh) pretreatment was given 30 min prior to the beginning of the relapse session. Nicotine (0.15 mg/kg s.c.) (Nic) or saline (Sal) priming was given at the end of the cue component. The cumulative response graphs illustrate that SB-277011-A produced a dose-dependent decrease in responding during the nicotine priming reinstatement session.
DISCUSSION
The present findings suggest that selective antagonism at DA D3 receptors by using the high-affinity antagonist SB-277011-A attenuated significantly nicotine-triggered reinstatement of nicotine-seeking behavior without affecting stable maintenance of nicotine self-administration per se. These results corroborate recent findings showing that SB-277011-A in the same dose range can block significantly cocaine-induced enhancement of brain stimulation reward, the development and expression of cocaine-induced conditioned place preference, and cocaine-triggered relapse to cocaine-seeking behavior (Vorel et al, 2002).
The available evidence shows that there is no clear consensus regarding the exact role of DA D1 or D2 agonists and antagonists in both cocaine- and nicotine-seeking behaviors. Nonselective DA D2/D3 agonists have been reported to mimic the effects of cocaine in drug discrimination (Spealman, 1996) and in cocaine self-administration paradigms (Parsons et al, 1996). Furthermore, the potency of such agents was shown to correlate significantly with their in vitro affinity and functional activity on DA D3, but not D2 receptors (Caine et al, 1997; Spealman, 1996). In the case of drug priming-induced relapse to cocaine seeking, evidence suggests that blockade of D2-like receptors by haloperidol (Ettenberg, 1990) or blockade of DA D1-like receptors by SCH 23390 (Norman et al, 1999) attenuates cocaine seeking. Furthermore, activation of D1-like receptors by direct agonists can inhibit cocaine seeking (Self et al, 1996,2000). Recent studies also report that the DA D1 antagonist SCH 23390 and the full agonist SKF 81297, but not the partial agonist SKF 38393, can attenuate cue-induced reinstatement of cocaine seeking (Alleweireldt et al, 2002). In addition, the DA D1-like agonists SKF 81297 and SKF 82958 attenuate priming-induced reinstatement of cocaine seeking in the Squirrel monkey in contrast with the D2 agonist R(−)-propylnorapomorphine hydrochloride (NPA) and the D3-preferring agonists PD 128,907 and 7-OH-DPAT, which failed to alter cocaine-triggered reinstatement of cocaine-seeking behavior (Khroyan et al, 2000).
In the case of nicotine, DA antagonists at both D1 and D2 receptors have been shown to produce a dose-dependent extinction-like intrasession reduction of responding for nicotine self-administration (Corrigall and Coen, 1991a,1991b). In addition, haloperidol reduces the number of both nicotine containing and denicotinized cigarettes, thus suggesting the involvement of non-nicotinic mechanisms (Brauer et al, 2001). However, haloperidol has also been shown to increase smoking behavior in both schizophrenic patients (McEvoy et al, 1995) and nonpsychiatric subjects (Caskey et al, 1999,2002; Dawe et al, 1995). Interestingly, DA agonist treatment can reduce responding for nicotine self-administration. For example, the DA D2 agonist bromocriptine seems to produce a longer latency to smoke and a longer intercigarette interval, a finding that is compatible with the hypothesis that bromocriptine can decrease smoking and nicotine intake (Caskey et al, 2002; Jarvik et al, 2000; Murphy et al, 2002). The recent study by Caskey et al (2002), which directly compared haloperidol and bromocriptine in a double-blind study, is the only within-subjects drug reversal design confirming that blockade of DA D2 receptors can increase, whereas activation of D2 receptors can decrease smoking behavior and nicotine intake in the same subjects. However, it is well known that both nausea and vomiting are common side effects of bromocriptine, a fact further supported by the observation that increased levels of nausea were related to decreased levels of smoking with higher doses of bromocriptine (Jarvik et al, 2000). Thus, the relative contributions of DA activity that are related to nausea or not remain to be fully assessed by future studies.
The present study supports the contention that selective blockade at DA D3 receptors does not affect nicotine self-administration per se, but rather selectively diminishes nicotine-triggered reinstatement of nicotine-seeking behavior. One may argue that the effects observed in the present study are partly mediated through an action of SB-277011-A at DA D2 receptors. This, however, is unlikely for the following reasons. First, in contrast with DA D2 antagonists, SB-277011-A does not produce any significant effect on both spontaneous locomotion and either amphetamine- or phencyclidine-induced hyperactivity (Reavill et al, 2000). It has been previously shown that the D3-preferring antagonist U-99194A can stimulate locomotor activity and potentiates both apomorphine- and amphetamine-induced hyperactivity (Waters et al, 1993). This compound, however, has only a 10-fold selectivity for DA D3 over D2 receptors (Audinot et al, 1998; Waters et al, 1993). Similarly, l-nafadotride can increase locomotor activity, but again this compound is only 10-fold selective for D3 over D2 receptors (Audinot et al, 1998; Sautel et al, 1995). In addition, the locomotor-stimulating effects of both l-nafadotride and U-99194A have been observed in DA D3 knockout mice, indicating that the stimulant properties of both compounds are unrelated to their D3 receptor blocking properties (Xu et al, 1999). Thus, these findings together with the majority of studies using DA D3 knockout mice support the contention that, contrary to DA D2 receptors, D3 receptors do not play an inhibitory role in locomotor activity in rodents. Second, in contrast with DA D2 receptor antagonists, SB-277011-A was shown not to be cataleptogenic at doses up to 78.8 mg/kg (Reavill et al, 2000). Third, again in contrast with DA D2 receptor antagonists, SB-277011-A failed to increase serum prolactin levels (hyperprolactinemia) (Reavill et al, 2000). Fourth, SB-277011-A reversed the DA D3-preferring agonist quinelorane-induced decrease in extracellular DA levels in the NAc (Reavill et al, 2000). In contrast, the effects of quinelorane in the dorsal striatum were not reversed even by doses of SB-277011-A up to 93 mg/kg, thus further reflecting regional differences in DA D3 receptor regulation of DA outflow. Fifth, lateral (right or left) shifts along the pulse frequency axis in the rate-frequency curve paradigm of the intracranial self-stimulation behavior has been considered a selective reward measure, whereas vertical shifts typically provide information about motor/performance capacity. It has been shown that DA D2 receptor antagonists typically produce a right shift in the rate-frequency curve (Miliaressis et al, 1986a,1986b; Stellar et al, 1983). Furthermore, the effectiveness of DA receptor antagonists to produce right shifts is correlated with their relative effectiveness in displacing DA D2 binding (Gallistel and Davis, 1983). If SB-277011-A would have any functionally significant DA D2 receptor antagonist properties, a right shift in the rate-frequency curve of brain stimulation reward would be observed in response to treatment with SB-277011-A alone. Rather, SB-277011-A produced a left shift in the same paradigm, thus further supporting the DA D3 antagonist properties of the effect (Vorel et al, 2002). Sixth, SB-277011-A did not produce conditioned place aversion (Vorel et al, 2002) in contrast with both the DA D3 agonists 7-OH-DPAT and PD-128907 and the partial D3 agonist BP-897 (Gyertyan and Gal, 2003). Altogether these results strengthen the suggestion that SB-277011-A diminishes nicotine-seeking behavior without altering the primary reinforcing effects of nicotine itself through a main action at DA D3 receptors.
It has been recently shown that BP 897 can reduce cocaine cue-induced hyperactivity (Le Foll et al, 2002) as well as cue-controlled cocaine-seeking behavior independently of any interaction with the reinforcing properties of cocaine itself (Pilla et al, 1999). In addition, BP 897 can attenuate the discriminative stimulus properties of both cocaine and amphetamine without being self-administered on its own (Beardsley et al, 2001). A similar profile has been observed with SB-277011-A (Di Ciano et al, 2003; Le Foll et al, 2002; Vorel et al, 2002). Recently, BP 897 has been shown to lack agonist activity and to have competitive antagonist properties at DA D3 receptors (Wood et al, 2000) in a real-time Cytosensor microphysiometry paradigm, which measures changes in extracellular acidification rates as a determinant of changes in cellular metabolic activity (Smart and Wood, 2000). These results thus raise the possibility that it is, in fact, the antagonist property of BP 897 that mediates its effects on cocaine-seeking behavior, further supporting the therapeutic utility of selective competitive antagonists at DA D3 receptors. Alternatively, one may suggest that SB-277011-A and BP-897 are acting through different mechanisms in different brain regions: one as an agonist and the other as a competitive antagonist. This hypothesis should be assessed by future studies. Also, the question of whether D3 receptor-mediated effects of quinelorane on DA outflow in the NAc are mediated via terminal autoreceptors or postsynaptic receptors is unclear and warrants further investigations.
One of the key remaining questions is related to the precise sites of action of DA D3 receptor ligands in regulating drug-seeking behavior. Increasing evidence converges onto the mesocortico-limbic DA system as a key player in the reinstatement of psychostimulant-seeking behavior triggered by drug priming. For example, direct infusion of DA into the NAc can reinstate cocaine seeking (Cornish and Kalivas, 2000), whereas microinfusions of the DA D2 antagonist fluphenazine into the medial prefrontal cortex (mPFC) can significantly attenuate cocaine-induced reinstatement (McFarland and Kalivas, 2001). Interestingly, direct microinfusion of the DA D2 antagonist haloperidol into the mPFC can decrease local DA levels whereas its systemic administration stimulates the percentage of burst firing and spikes per burst of VTA DA neurons antidromically identified from the mPFC (Gessa et al, 2000). In contrast, SB-277011-A does not modify basal DA neurotransmission in the NAc, but can reverse quinelorane-induced decreases in extracellular DA levels in the same brain area (Reavill et al, 2000). We have recently shown that the acute systemic administration of SB-277011-A can increase extracellular DA levels in the rat anterior cingulate cortex (ACg) (Lacroix et al, 2003). Interestingly, the projections from the ACg terminate more medially and extend further ventrally to include the core of the NAc (Ding et al, 2001). It has been shown that lesions of the ACg produce persistent responding for cocaine when responding is no longer under the control of contingent presentations of cocaine-associated cues (Weissenborn et al, 1997). Furthermore, lesions of the core subregion of the NAc impair the acquisition of cocaine seeking under a second-order schedule (Ito et al, 2001). The question of whether and how the effects of SB-277011-A on DA in the ACg are related to reinstatement of drug-seeking behaviors warrants further investigations.
Priming injections of cocaine can increase extracellular levels of DA in both the NAc (Neisewander et al, 1996) and amygdala (Tran-Nguyen et al, 1998) and can increase Fos protein expression in the VTA as well as caudate-putamen, central and lateral nuclei of the amygdala, and ACg (Neisewander et al, 2000). In addition, priming injections of D-amphetamine produce increases in DA chronoamperometric signals in the NAc (Di Ciano et al, 2001). These results point again towards DA neurotransmission in the NAc as an important factor in drug priming-induced reinstatement of drug-seeking behavior. In addition to the NAc and ACg, the basolateral amygdala (BLA) has also been shown to play a key role in conditioned reinforcement (Everitt et al, 1999) and cue-controlled cocaine seeking (Whitelaw et al, 1996). Thus, the ACg along with the BLA both contribute to associative mechanisms controlling cocaine seeking. These findings are also in line with functional neuroimaging studies (Childress et al, 1999; Grant et al, 1996; O'Brien et al, 1998). Finally, another site of interest is the ICjM that shows extensive expression of the DA D3 receptor (Diaz et al, 2000; Sokoloff et al, 1990). Although few studies have investigated the potential role of ICjM in brain-reward mechanisms, two pieces of evidence are worth mentioning. One study (Nakajima and Patterson, 1997) claimed that DA D2, but not D3 or D4, receptors in the NAc, IcjM, and VTA are critically involved in the rewarding effects of intracranial self-stimulation behavior. However, although these authors used convincing drugs for targeting the DA D2 receptor, they used (+)-UH232, which has only 10- to 20-fold selectivity for D3 over D2 receptors, to study the potential role of DA D3 receptors. Thus, these effects can be explained by D2-mediated rather than D3-mediated mechanisms and are consistent with the results obtained with raclopride and haloperidol. Furthermore, these observations with (+)-UH232 are not in line with those of Kling-Petersen et al (1994). The second study involved 2-deoxyglucose autoradiographic measurements during withdrawal from cocaine self-administration (Hammer et al, 1993). In these studies, metabolic activity was reduced during withdrawal from cocaine in the NAc, olfactory tubercle, ICjM, piriform cortex, ACg, rostral caudate-putamen, entopeduncular nucleus, lateral hypothalamus, as well as somatosensory, auditory, and motor cortices. Furthermore, the results suggested that the longer the withdrawal period (72 vs 6 h after binge exposure), the more severe the effect on metabolic rate for glucose. Again, the potential role of the DA D3 receptor in the ICjM against drug priming-induced reinstatement of drug-seeking behavior remains to be fully investigated.
CONCLUSIONS
The results of the present study show that DA D3 receptors play an important role in nicotine-triggered relapse to nicotine-seeking behavior without affecting stable maintenance of nicotine self-administration. Together with a growing body of evidence in the literature, the present findings also suggest that the neuronal processes mediating drug-induced reinstatement may be different, at least in part, from those involved in the primary reinforcing effects of the drug. Thus, efficacy in reducing drug self-administration per se is not necessarily predictive of efficacy in preventing relapse triggered by drug priming, cues or stress. These results point towards selective modulation of DA D3 receptors as a valuable pharmacotherapeutic strategy for the management of biobehavioral dysregulations associated with nicotine dependence and addiction. Obviously, the precise site of action of SB-277011-A is still unclear and the assessment of the central sites of action of DA D3 antagonists will be an important goal of future studies.
References
Alleweireldt AT, Weber SM, Kirschner KF, Bullock BL, Neisewander JL (2002). Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 159: 284–293.
Ashby Jr CR, Minabe Y, Stemp G, Hagan JJ, Middlemiss DN (2000). Acute and chronic administration of the selective D(3) receptor antagonist SB-277011-A alters activity of midbrain dopamine neurons in rats: an in vivo electrophysiological study. J Pharmacol Exp Ther 294: 1166–1174.
Audinot V, Newman-Tancredi A, Gobert A, Rivet JM, Brocco M, Lejeune F et al (1998). A comparative in vitro and in vivo pharmacological characterization of the novel dopamine D3 receptor antagonists (+)-S 14297, nafadotride, GR 103,691 and U 99194. J Pharmacol Exp Ther 287: 187–197.
Austin NE, Baldwin, Cutler L, Deeks N, Kelly PJ, Nash M et al (2001). Pharmacokinetics of the novel, high-affinity and selective dopamine D3 receptor antagonist SB-277011 in rat, dog and monkey: in vitro/in vivo correlation and the role of aldehyde oxidase. Xenobiotica 31: 677–686.
Balfour DJ, Wright AE, Benwell ME, Birrell CE (2000). The putative role of extra-synaptic mesolimbic dopamine in the neurobiology of nicotine dependence. Behav Brain Res 113: 73–83.
Beardsley PM, Sokoloff P, Balster RL, Schwartz JC (2001). The D3R partial agonist, BP897, attenuates the discriminative stimulus effects of cocaine and D-amphetamine and is not self-administered. Behav Pharmacol 12: 1–11.
Berridge KC, Robinson TE (1998). What is the role of dopamine in reward: hedonic impact, reward learning or incentive salience. Brain Res Rev 28: 309–369.
Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC (1991). Localisation of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res 564: 203–219.
Brauer LH, Cramblett MJ, Paxton DA, Rose JE (2001). Halperidol reduces smoking of both nicotine-containing and denicotinized cigarettes. Psychopharmacology 159: 31–37.
Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA et al (2001). Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav 70: 515–530.
Caine SB, Koob GF, Parsons LH, Everitt BJ, Schwartz JC, Sokoloff P (1997). D3 receptor test in vitro predicts decreased cocaine self-administration in rats. NeuroReport 8: 2373–2377.
Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J et al (2002). Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. J Neurosci 22: 2977–2988.
Caskey NH, Jarvik ME, Wirshing WC (1999). The effects of dopaminergic D2 stimulation and blockade on smoking behavior. Exp Clin Psychopharmacol 7: 72–78.
Caskey NH, Jarvik ME, Wirshing WC, Madsen DC, Iwanoto-Schaap PN, Eisenberger NI et al (2002). Modulating tobacco smoking rates by dopaminergic stimulation and blockade. Nicotine Tob Res 4: 259–266.
Chiamulera C, Rezzaghi F, Ardizzon M, Motta C, Marcon C, Tessari MA (1998). A novel model to study relapse to nicotine-seeking behaviour in rats. Arch Pharmacol 358: R578.
Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP (1999). Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156: 11–18.
Cornish JL, Kalivas PW (2000). Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci 20: RC89.
Corrigall WA, Coen KM (1991a). Cocaine self-administration is increased by both D1 and D2 dopamine antagonists. Pharmacol Biochem Behav 39: 799–802.
Corrigall WA, Coen KM (1991b). Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology (Berl) 104: 171–176.
Corrigall WA, Coen KM, Zhang J, Adamson KL (2002). Pharmacological manipulations of the pedunculopontine tegmental nucleus in the rat reduce self-adminitration of both nicotine and cocaine. Psychopharmacology 160: 198–205.
Dawe S, Gerada C, Russell MA, Gray JA (1995). Nicotine intake in smokers increases following a single dose of haloperidol. Psychopharmacology (Berl) 117: 110–115.
Deroche-Gamonet V, Piat F, Le Moal M, Piazza PV (2002). Influence of cue-conditioning on acquisition, maintenance and relapse of cocaine intravenous self-administration. Eur J Neurosci 15: 1363–1370.
Diaz J, Levesque D, Lammers CH, Grffon N, Martres MP, Schwartz JC et al (1995). Phenotypical characterization of neurons expressing the dopamine D3 receptor in the rat brain. Neuroscience 65: 731–745.
Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz JC et al (2000). Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci 20: 8677–8684.
Di Chiara G, Imperato A (1988). Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85: 5274–5278.
Di Chiara G (1995). The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug Alcohol Depend 28: 95–137.
Di Ciano P, Blaha CD, Phillips AG (2001). Changes in dopamine efflux associated with extinction, CS-induced and D-amphetamine-induced reinstatement of drug-seeking behaviour by rats. Behav Brain Res 120: 147–158.
Di Ciano P, Underwood R, Hagan JJ, Everitt BJ (2003). Attenuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-277011-A. Neuropsychopharmacology 28: 329–338.
Ding DCD, Gabbott PLA, Totterdell S (2001). Differences in the laminar origin of projections from the medial prefrontal cortex to the nucleus accumbens shell and core regions in the rat. Brain Res 917: 81–89.
Ettenberg A (1990). Haloperidol prevents the reinstatement of amphetamine-rewarded runway responding in rats. Pharmacol Biochem Behav 36: 635–638.
Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW (1999). Associative processes in addiction and reward: the role of amygdala-ventral striatal subsystems. Ann NY Acad Sci 877: 412–438.
Fagerstrom KO, Schneider NG (1989). Measuring nicotine dependence: a review of the Fagerstrom Tolerance Questionnaire. J Behav Med 12: 159–182.
Gallistel CR, Davis AJ (1983). Affinity for the dopamine D2 receptor predicts neuroleptic potency in blocking the reinforcing effect of MFB stimulation. Pharmacol Biochem Behav 19: 867–872.
Gessa GL, Devoto P, Diana M, Flore G, Melis M, Pistis M (2000). Dissociation of haloperidol, clozapine, and olanzapine effects on electrical activity of mesocortical dopamine neurons and dopamine release in the prefrontal cortex. Neuropsychopharmacology 22: 642–649.
Goeders NE, Clampitt DM (2002). Potential role for the hypothalamus–pituitary–adrenal axis in the conditioned reinforcer-induced reinstatement of extinguished cocaine seeking in rats. Psychopharmacology 161: 222–232.
Grant S, London ED, Newlin DB, Villemagne VL, Xiang L, Contoreggi C et al (1996). Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA 93: 12040–12045.
Gurevich EV, Joyce JN (1999). Distribution of dopamine D3 receptor expressing neurons in the human forebrain: comparison with D2 receptor expressing neurons. Neuropsychopharmacology 20: 60–80.
Gyertyan I, Gal K (2003). Dopamine D3 receptor ligands show place conditioning effect but do not influence cocaine-induced place preference. NeuroReport 14: 93–98.
Haile CN, Kosten TA (2001). Differential effects of D1- and D2-like compounds on cocaine self-administration in Lewis and Fischer 344 inbred rats. J Pharmacol Exp Ther 299: 509–518.
Hall H, Halldin C, Dijkstra D, Wiltröm H, Wise LD, Purgsley TA et al (1996). Autoradiographic localisation of D3-dopamine receptors in the human brain using the selective D3-dopamine receptor agonist (+)-[3H]PD128,907. Psychopharmacology 128: 240–247.
Hammer RP, Pires WS, Markou A, Koob GF (1993). Withdrawal following cocaine self-administration decreases regional cerebral metabolic rate in critical brain reward regions. Synapse 14: 73–80.
Highfield DA, Mead AN, Grimm JW, Rocha BA, Shaham Y (2002). Reinstatement of cocaine seeking in 129X1/SvJ mice: effects of cocaine cues and food deprivation. Psychopharmacology 161: 417–424.
Hummel M, Unterwald EM (2002). D1 dopamine receptor: a putative neurochemical and behavioral link to cocaine action. J Cell Physiol 191: 17–27.
Ito R, Robbins TW, Everitt BJ (2001). Dissociation in the effects of lesions of the nucleus accumbems core and shell on the acquisition of a second-order schedule of cocaine reinforcement. Soc Neurosci Abstr 27: 443.9.
Jarvik ME, Caskey NH, Wirshing WC, Madsen DC, Iwanoto-Schaap PN, Elins JL et al (2000). Bromocriptine reduces cigarette smoking. Addiction 95: 1173–1183.
Khroyan TV, Barrett-Larimore RL, Rowlett JK, Spealman RD (2000). Dopamine D1- and D2-like receptor mechanisms in relapse to cocaine-seeking behavior: effects of selective antagonists and agonists. J Pharmacol Exp Ther 294: 680–687.
Kling-Petersen T, Ljung E, Svensson K (1994). The pre-ferential dopamine autoreceptor antagonist (+)-UH232 antagonizes the positive reinforcing effects of cocaine and d-amphetamine in the ICSS paradigm. Pharmacol Biochem Behav 49: 345–351.
Koob GF, Le Moal M (1998). Drug abuse: hedonic homeostatic dystregulation. Science 278: 52–58.
Lacroix LL, Hows MA, Shah AJ, Hagan JJ, Heidbreder CA (2003). Antagonism at dopamine D3 receptors enhances monoaminergic and cholinergic neurotransmission in the rat anterior cingulate cortex. Neuropsychopharmacology, in press.
Landwehrmeyer B, Mengod G, Palacios JM (1993). Dopamine D3 receptor mRNA and binding sites in human brain. Brain Res Mol Brain Res 18: 187–192.
Le Foll B, Diaz J, Sokoloff P (2003). Increased dopamine D3 receptor expression accompanying behavioural sensitization to nicotine in rats. Synapse 47: 176–183.
Le Foll B, Frances H, Diaz J, Schwartz J-C, Sokoloff P (2002). Role of the dopamine D3 receptor in reactivity to cocaine-associated cues in mice. Eur J Neurosci 15: 2016–2026.
Mash DC, Staley JK (1999). D3 dopamine and kappa opioid receptor alterations in human brain of cocaine-overdose victims. Ann NY Acad Sci 877: 507–522.
McEvoy JP, Freudenreich O, Levin ED, Rose JE (1995). Haloperidol increases smoking in patients with schizophrenia. Psychopharmacology (Berl) 119: 124–126.
McFarland K, Kalivas PW (2001). The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 21: 8655–8663.
Miliaressis E, Malette J, Coulombe D (1986a). The effects of pimozide on the reinforcing efficacy of central gray stimulation in the rat. Behav Brain Res 21: 95–100.
Miliaressis E, Rompre PP, Laviolette P, Philippe L, Coulombe D (1986b). The curve-shift paradigm in self-stimulation. Physiol Behav 37: 85–93.
Murphy MF, Hey K, Johnstone E, Munafo M, Walton R, Willis B et al (2002). Bromocriptine use is associated with decreased smoking rates. Addict Biol 7: 325–328.
Murray AM, Ryoo HL, Gurevich E, Joyce JN (1994). Localization of dopamine D3 receptors to mesolimbic and D2 receptors to mesostriatal regions of human forebrain. Proc Natl Acad Sci USA 91: 11271–11275.
Nakajima S, Patterson RL (1997). The involvement of dopamine D2 receptors, but not D3 or D4 receptors, in the rewarding effect of brain stimulation in the rat. Brain Res 760: 74–79.
Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF (2000). Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci 20: 798–805.
Neisewander JL, O'Dell LE, Tran-Nguyen LT, Castadena E, Fuchs RA (1996). Dopamine overflow in the nucleus accumbens during extinction and reinstatement of cocaine self-administration behavior. Neuropsychopharmacology 15: 506–514.
Norman AB, Norman MK, Hall JF, Tsibulsky VL (1999). Priming threshold: a novel quantitative measure of the reinstatement of cocaine self-administration. Brain Res 831: 165–174.
O'Brien CP, Childress AR, Ehrman RN, Robbins SJ (1998). Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol 12: 15–22.
Parsons LH, Caine SB, Sokoloff P, Schwartz JC, Koob GF, Weiss F (1996). Neurochemical evidence that postsynaptic nucleus accumbens D3 receptor stimulation enhances cocaine reinforcement. J Neurochem 67: 1078–1089.
Paterson NE, Markou A (2002). Increased GABA neurotransmission via administration of gamma-vinyl GABA decreased nicotine self-administration in the rat. Synapse 44: 252–253.
Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG et al (1999). Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature (Lond) 400: 371–375.
Reavill C, Taylor SG, Wood MD, Ashmeade T, Austin NE, Avenell KY et al (2000). Pharmacological actions of a novel, high-affinity, and selective human dopamine D3 receptor antagonist, SB-277011-A. J Pharmacol Exp Ther 294: 1154–1165.
Sautel F, Griffon N, Sokoloff P, Schwartz JC, Launay C, Simon P et al (1995). Nafadotride, a potent preferential dopamine D3 receptor antagonist, activates locomotion in rodents. J Pharmacol Exp Ther 275: 1239–1246.
Schenk S, Partridge B (1999). Cocaine-seeking produced by experimenter-administered drug injections: dose–effect relationships in rats. Psychopharmacology (Berl) 147: 285–290.
Segal DM, Moraes CT, Mash DC (1997). Up-regulation of D3 dopamine receptor mRNA in the nucleus accumbens of human cocaine fatalities. Brain Res Mol Brain Res 45: 335–339.
Self DW, Barnhart WJ, Lehman DA, Nestler EJ (1996). Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science (Wash, DC) 271: 1586–1589.
Self DW, Genova LM, Hope BT, Barnhart WJ, Spencer JJ, Nestler EJ (1998). Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self-administration and relapse of cocaine-seeking behavior. J Neurosci 18: 1848–1859.
Self DW, Karanian DA, Spencer JJ (2000). Effects of the novel D1 dopamine receptor agonist ABT-431 on cocaine self-administration and reinstatement. Ann NY Acad Sci 909: 133–144.
Shafer RA, Levant B (1998). The D3 dopamine receptor in cellular and organismal function. Psychopharmacology 135: 1–16.
Shoaib M, Stolerman IP (1999). Plasma nicotine and cotinine levels following intravenous nicotine self-administration in rats. Psychopharmacology 143: 318–321.
Smart D, Wood MD (2000). Cytosensor techniques for examining signal transduction of neurohormones. Biochem Cell Biol 78: 281–288.
Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC (1990). Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature 347: 146–151.
Spealman RD (1996). Dopamine D3 receptor agonists partially reproduce the discriminative stimulus effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther 278: 1128–1137.
Staley JK, Mash DC (1996). Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci 16: 6100–6106.
Stellar JR, Kelly A, Corbett D (1983). Effects of peripheral and central dopamine blockade on lateral hypothalamic stimulation reward: evidence for both reward and motor deficits. Pharmacol Biochem Behav 18: 433–442.
Stemp G, Ashmeade T, Branch CL, Hadley MS, Hunter AJ, Johnson CN et al (2000). Design and synthesis of trans-N-[4-[2-(6-cyano-1,2,3, 4-tetrahydroisoquinolin-2-yl)ethyl]cyclohexyl]-4-quinolinecarboxamide (SB-277011): a potent and selective dopamine D3 receptor antagonist with high oral bioavailability and CNS penetration in the rat. J Med Chem 43: 1878–1885.
Suzuki M, Hurd YL, Sokoloff P, Schwartz J-C, Sedvall G (1998). D3 dopamine receptor mRNA is widely expressed in the human brain. Brain Res 779: 58–74.
Tran-Nguyen TL, Baker DA, Grote KA, Solano J, Neisewander JL (1998). Serotonin depletion attenuates cocaine-seeking behavior in rats. Psychopharmacology 146: 60–66.
Vorel SR, Ashby Jr CR, Paul M, Liu X, Hayes R, Hagan JJ et al (2002). Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci 22: 9595–9603.
Waters N, Lofberg L, Haadsma-Svensson S, Svensson K, Sonesson C, Carlsson A (1994). Differential effects of dopamine D2 and D3 receptor antagonists in regard to dopamine release, in vivo receptor displacement and behaviour. J Neural Transm Gen Sect 98: 39–55.
Waters N, Svensson K, Haadsma-Svensson SR, Smith MW, Carlsson A (1993). The dopamine D3-receptor: a postsynaptic receptor inhibitory on rat locomotor activity. J Neural Transm 94: 11–19.
Weissenborn R, Robbins TW, Everitt BJ (1997). Effects of medial prefrontal or anterior cingulate cortex lesions on responding for cocaine under fixed-ratio and second-order schedules of reinforcement in rats. Psychopharmacology 134: 242–257.
Whitelaw RB, Markou A, Robbins TW, Everitt BJ (1996). Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacology 127: 213–224.
Wise RA, Rompre P-P (1989). Brain dopamine and reward. Annu Rev Psychol 40: 191–225.
Wood MD, Boyfield I, Nash DJ, Jewitt FR, Avenell KY, Riley GJ (2000). Evidence for antagonist activity of the dopamine D3 receptor partial agonist, BP 897, at human dopamine D3 receptor. Eur J Pharmacol 407: 47–51.
Xu M, Koeltzow TE, Cooper DC, Tonegawa S, White FJ (1999). Dopamine D3 receptor mutant mice exhibit identical responses to putative D3 receptor-selective agonists and antagonists. Synapse 31: 210–215.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andreoli, M., Tessari, M., Pilla, M. et al. Selective Antagonism at Dopamine D3 Receptors Prevents Nicotine-Triggered Relapse to Nicotine-Seeking Behavior. Neuropsychopharmacol 28, 1272–1280 (2003). https://doi.org/10.1038/sj.npp.1300183
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300183
Keywords
This article is cited by
-
Exploring the role of the Ser9Gly (rs6280) Dopamine D3 receptor polymorphism in nicotine reinforcement and cue-elicited craving
Scientific Reports (2020)
-
Dopamine D3R antagonist VK4-116 attenuates oxycodone self-administration and reinstatement without compromising its antinociceptive effects
Neuropsychopharmacology (2019)
-
Dopamine and addiction: what have we learned from 40 years of research
Journal of Neural Transmission (2019)
-
In Vivo Imaging of Cerebral Dopamine D3 Receptors in Alcoholism
Neuropsychopharmacology (2014)
-
Effects of Chronic Varenicline Treatment on Nicotine, Cocaine, and Concurrent Nicotine+Cocaine Self-Administration
Neuropsychopharmacology (2014)